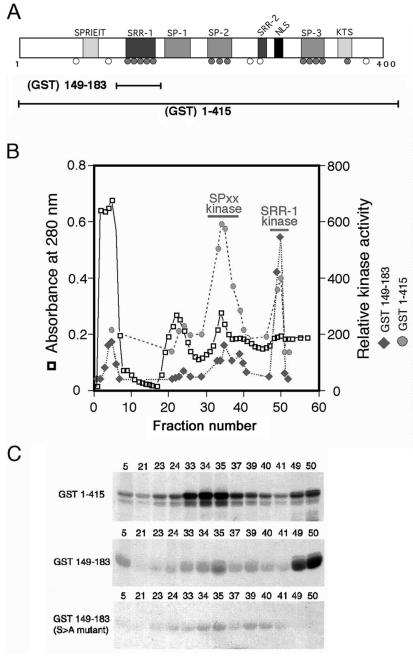
FIG. 1.
NFAT1 is phosphorylated by two distinct kinases. (A) N-terminal regulatory domain of NFAT1, showing the conserved serine motifs and relative positions of phosphorylated serine residues identified in NFAT1 from resting T cells. Shaded circles, conserved phosphoserines that become dephosphorylated upon stimulation; open circles, nonconserved or constitutively phosphorylated serines. GST(149-183) includes only the region containing the NFAT1 SRR-1 motif, while GST(1-415) encompasses the entire NFAT1 regulatory domain. (B) Fractionation of T-cell lysates over a cation-exchange column reveals two separate peaks of kinase activity when GST(1-415) is used as a substrate (shaded circles). Assay of the same fractions using GST(149-183) as a substrate shows that only one of these peaks contains an activity that is able to phosphorylate the SRR-1 motif (solid diamonds). (C) Autoradiograph of kinase assays from the fractionation shown in panel B. NFAT1 kinase activity was assessed by incubating an aliquot from each fraction with either GST(1-415) or GST(149-183) in the presence of [γ-32P]ATP. For GST(1-415), there are two peaks of activity: fractions 33 to 37 and fractions 49 and 50 (top panel). For GST(149-183), only the activity present in fractions 49 and 50 is capable of efficiently phosphorylating the fusion protein (center panel). Mutation of the conserved serines in the SRR-1 motif to alanine completely abolishes phosphorylation of GST(149-183) by the kinase activity present in fractions 49 and 50 (bottom panel).