Abstract
Currently, fragile X syndrome (FXS), caused by a trinucleotide expansion (> 200 CGG) in the FMR1 gene, is not included in newborn screening panels in the United States, as it does not meet the standards for recommendation. Although in the past few years FXS has met many of the criteria for population screening, and studies have shown that newborn screening (NBS) for FXS is feasible, the idea is still controversial and the debate is open. The recent advances in genomic testing, and groundbreaking advances in targeted treatment for FXS, have been challenging the dogma and principle of the national NBS program: “screen only if you can intervene.” Arguments in favor of NBS include benefits of early intervention and follow up for the identified baby, which would justify NBS even in the absence of medical benefit to the child. In addition, the extended family members may benefit from genetic and reproductive counseling, informed decision-making before a subsequent pregnancy, and access to treatment and services. However, communicating the results and the potential consequences to families is a challenge, and could lead to a heavy psychosocial burden. A controversial issue is the identification of premutation carriers (55–200 CGG), because, not only it can lead to information on the reproductive possibility of having a child with FXS, but also leads to information about personal health risks associated with the premutation. Yet, knowledge of carrier status could stimulate and encourage lifestyle changes and preventive measures likely to reduce the risk of medical problems reported in premutation carrier.
If NBS for FXS is developed, it must be carried out with clear awareness the potential impact on the lives of the children, and it should be done after counseling and parents’ informed consent. Importantly, the infrastructure to support testing, counseling, treatment, and follow-up will have to be made available to the families.
Mandatory newborn screening programs in the United States, using a simple blood test on blood spot cards, have been designed as a public health program to identify a number of rare conditions that are not apparent at birth. If not diagnosed at birth and not treated, these disorders will cause significant disability or death. Indeed, since the development of the screening test for phenylketonuria using bloodspots dried onto a filter paper card, the newborn screening health program has been a national priority designed to save or improve the lives of affected babies. Although newborn screening (NBS) has primarily implemented a short-term follow up process, the need for long-term follow up has been recently recognized as essential for optimal treatment and comprehensive care. Every state in US has mandated independent newborn screening programs, which includes different conditions based on the severity of the condition, the availability of effective treatment, and the cost of the test. The NBS program has seen a rapid increase in the number of known genetic conditions screened, particularly due to technological developments, which have led to increased accuracy and reduced costs of the tests. Conversely, expanded screening has raised a number of concerns, including lack of evidence-based research for treatment, shortage of follow-up services, unclear financial responsibilities, and ethical, legal, and social implications of situations such as disclosing carrier status or susceptibility to future disease.
History of newborn screening in fragile X syndrome
Fragile X syndrome, the most well-known and most common single-gene cause of inherited intellectual disabilities (ID) and autism, is due to a trinucleotide CGG-repeat expansion (>200 CGG repeats; full mutation) in the 5′ untranslated region of the fragile X mental retardation 1 (FMR1) gene. Epigenetic modification of the CGG-rich region turns off the gene, which results in the absence or deficit of the encoded product, FMRP, leading to reduced synaptic plasticity, which is important for learning and memory.1 Although FXS is the most common cause of inherited ID, the mean age of diagnosis for males is approximately 36 months of age and is higher in females. Many families have a second child with FXS before the first one has been diagnosed.2
Newborn screening (NBS) for fragile X syndrome (FXS) is controversial; FXS was not recommended for inclusion in the panel of conditions for newborn screening, as described in the 2006 American College of Medical Genetics report,3 partly because there was no medical advantage for early detection. However, in the past few years, reports from large screening studies demonstrating the technical feasibility of widespread testing, the benefits from genetic counseling, and advances in treatment, may change this scenario.
The spectrum of clinical involvement
Individuals with a full mutation present with a constellation of involvement, including ID, autism spectrum disorder, social anxiety and withdrawal, language deficits, hyperactivity, aggression, and self-injurious behaviors, in addition to physical features such as hyperextensible finger joints, prominent ears, and macroorchidism in puberty.4
Premutation FMR1 alleles, harboring between 55 and 200 CGG repeats, are unstable and can expand to a full mutation within one generation; consequently, women premutation carriers are at increased risk of having children with FXS. An expanding number of medical disorders, including the well-established fragile X-associated tremor/ataxia syndrome (FXTAS) and fragile X-associated primary ovarian insufficiency (FXPOI), occur in some carriers of premutation alleles; in the premutation population, these problems include seizures (13%), hypertension (60%), neuropathy (40%), migraines (30%), sleep apnea (30%), immune-mediated problems (44%), neurological and psychiatric problems (60%), autism spectrum disorder (ASD 10%), and attention deficit hyperactivity disorder (ADHD, 40%). Although ADHD and ASD occur in childhood, the other disorders have been found throughout the lifespan of carriers, and some commonly occur before the onset of FXTAS.5 Studies in the premutation mouse model have demonstrated developmental problems, with the degree of involvement correlating with CGG-repeat number and FMR1 mRNA levels6, in addition to ovarian abnormalities, involving both oocytes and granulosa cells,7 mitochondrial dysfunction,8 and altered calcium regulation.9
Individuals who carry an intermediate (or gray zone) allele of 45 to 54 CGG repeats, which are unstable when transmitted across generations, may be at risk for FXTAS and FXPOI.5 However, the role of gray zone alleles is currently unclear, and future research is necessary to help our understanding of the potential impact on human health.
Population screening and prevalence
Estimated prevalence of both full mutation and premutation individuals varies depending on the geographical area and population assessed. In the general population, the range for full mutation alleles is 1:2,500 to 1:8,000 of females, and approximately 1:5,000 of males. The prevalence of premutation alleles ranges from 1:130–256 of females, and 1:250–810 of males in the general population. Although CGG-allele size distribution appears to be similar between genders, and among ethnic and racial groups, differences in the prevalence of expanded alleles have been reported among different populations.10
While molecular characterization of the FMR1 gene is complex because of the presence of the high CG-rich sequence, recent advances in genetic testing methods have led to the10 development of efficient methodologies that have enabled several PCR-based screening studies, using DNA isolated from either whole blood or from blood-spot cards. (Fig. 1) These large-scale, population-based screening studies span the entire spectrum of FMR1 mutations in the United States.10
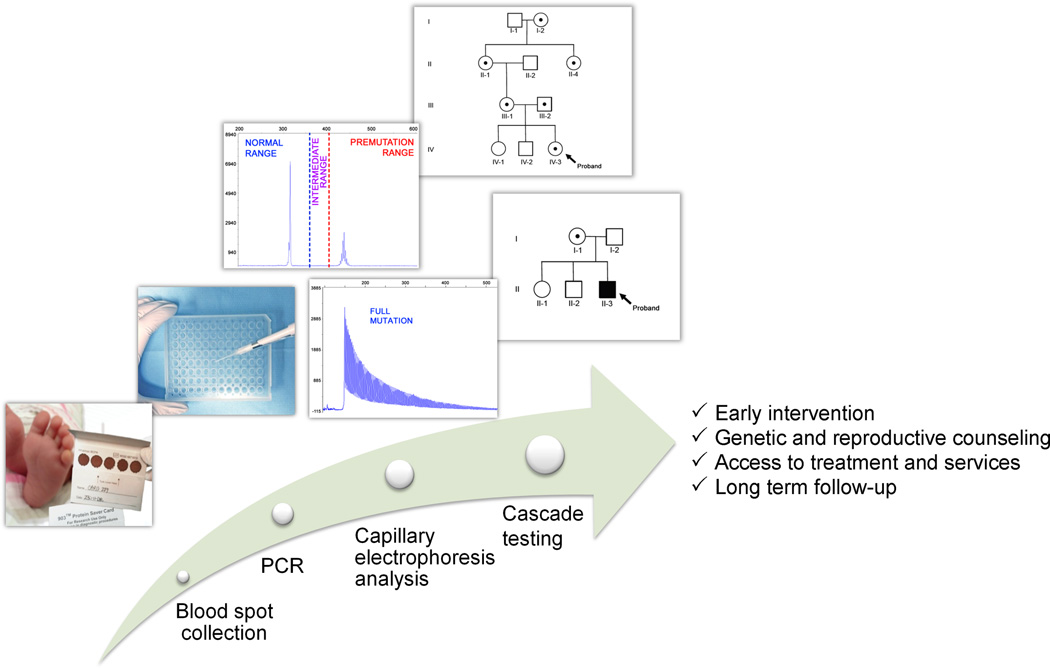
Figure 1.
Diagram of the newborn-screening process. From the left, blood collected on a bloodspot card from a heel prick is used to screen newborns for Fragile X. Isolation of DNA, PCR and Capillary Electrophoresis analysis allow measurement of CGG-repeat allele size. The identification of individuals with an FMR1 expanded allele can lead to cascade testing of extended family members. The benefits of cascade testing include early intervention, genetic and reproductive counseling, access to behavioral and pharmacological treatment, and long-term follow-up services.
Of these US screening studies, 8 reported on newborn screening, 6 of which were conducted on males only, and 2 on both males and females (Table 1). The largest US pilot study of NBS for all FMR1 mutations demonstrated that using blood spots is possible and reliable, and suggested that the prevalence of the premutation, particularly in males, is higher than had been previously reported,10 in agreement with rates from a large population-based study of approximately 20,000 male and female adults.11 Interestingly, most of the identified carriers had a very low CGG-repeat numbers, with more than 70% having an allele of <70 CGG repeats, 10 representing a relatively lower rate of reproductive and neurological risk problems than those with longer premutation expansions alleles with a higher CGG-repeat number. Additionally, Tassone et al. documented differences in prevalence rates among various ethnic groups; the authors found a lower premutation prevalence in African-American males (1:780), compared to Caucasian males (1:358), and males of Hispanic ethnicity (1:595).10
Table 1.
Prevalence data obtained from newborn screening studiesa
| Location | Ethnicity | Number Tested |
Gender | Genotype | CGG Range |
Prevalence |
|---|---|---|---|---|---|---|
| Georgia, USA | 45% Caucasian | 36,124 | Male | Full | >200 | - |
| 30% African American | ||||||
| 15% Hispanic | ||||||
| 2% Asian | ||||||
| 2% Multicultural | ||||||
| 1% American Indian | ||||||
| 5% Unknown | ||||||
| Taiwan | Asian | 4,843 | Male | Gray | 40–54 | - |
| Pre | 55–200 | - | ||||
| Full | >200 | - | ||||
| Canada | Canadian | 1,000 | Male | Gray | 40–60 | - |
| 1,000 | Female | Pre | >60 | - | ||
| Spain | Hispanic | 5,267 | Male | Gray | 45–54 | 1/26 |
| Pre | 55–200 | 1/251 | ||||
| Full | >200 | 1/2633 | ||||
| Catalan, Spain | Hispanic | 5,000 | Male | Gray | 53–55 | 1/449 |
| Pre | 56–200 | 1/1233 | ||||
| Full | >200 | 1/2466 | ||||
| South Carolina, USA | NAb | 1,459 | Male | Pre | 55–200 | 1/730 |
| Full | >200 | 1/730 | ||||
| Taiwan | Asian | Gray | 45–54 | 1/143 | ||
| 10,046 | Male | Pre | 55–200 | 1/1674 | ||
| Full | >200 | - | ||||
| USA | 29% Caucasian | 7,312 | Male | Gray | 45–54 | 1/112 |
| 25% Hispanic | Pre | 55–200 | 1/430 | |||
| 22% African American | Full | >200 | - | |||
| 6% Asian | 6,895 | Female | Gray | 45–54 | 1/66 | |
| 9% Others | Pre | 55–200 | 1/209 |
Adapted from Tassone et al.10;
racial data not collected for this study
Understanding the need for NBS
The Tassone et al. pilot NBS study10 involved a voluntary informed consent process whereby families were offered the opportunity to participate in NBS for the FMR1 mutations. Remarkably, investigators found that a majority of families consented to the study; the acceptance rate was approximately 60% to 76%, depending on the investigation site. Although a recent Australian study1 reported a much higher rate of participation, overall findings indicate a high level of maternal acceptance and voluntary support of NBS for fragile X mutations. The newborn screening study identified a cohort of babies with the premutation, generating an unselected premutation population that could be followed developmentally.10 Preliminary data presented at the 1st International Conference on the FMR1 Premutation12,13 suggested that premutation newborns present early developmental problems, particularly in social/emotional domains and behavior compared to age-matched controls, and show evidence of developmental problems in visual perception. Although very preliminary, the data emphasizes the great need to study the processing capabilities and developmental trajectories of infants and toddlers carrying a premutation.
The Tassone et al.10 pilot study was designed to determine the feasibility of screening in anticipation of a possible future scenario of NBS for fragile X mutations and in this respect it succeeded. However, because it identified both premutation carriers and gray zone alleles, the study findings have raised questions about carrier screening.
Should we screen for the premutation?
Carrier screening is usually seen in the context of reproductive healthcare whereby the outcome may inform parents about reproductive options and family planning. In the case of FXS, the identification of carriers can not only advise on the reproductive risk of having a child with FXS, but may also inform about personal health risks; the latter raises concerns for genetic counseling regarding the potential to develop, for example, late-onset neurological problems (FXTAS), anxiety, depression, or other medical conditions associated with the FMR1 premutation.5
The identification of the proband through NBS also raises the need for cascade testing of other family members, and subsequent identification of individuals who may be affected by the premutation or full mutation, which can present ethical and legal issues because family members did not directly consent to the screening. Identifying a baby with expanded alleles could lead to increased anxiety, stress, and depression in parents and related family members. However, a recent study12 of parents of babies with expanded alleles showed that the quality of the parents’ lives, as defined by scores from measures of anxiety, depression, and stress, was not more affected compared to those without an affected infant who agreed to participate in NBS. If accepted, screening for fragile X should be voluntary, and, therefore, involve a consent process, which could overwhelm parents and burden hospitals. However, recently reported data does not indicate a reduced participation in screening programs because of these concerns; indeed, a high participation rate was observed.10,12,14
The identification of gray zone alleles is problematic because the research regarding clinical involvement is very limited and the prevalence of that expansion is very high.10,11 Although abnormal molecular phenotypes (higher FMR1 mRNA and lower FMRP expression levels) have been reported, and some studies have indicated a potential risk for both FXTAS and FXPOI in individuals carrying a gray zone allele, more studies of an unselected sample of gray-zone carriers are necessary to understand the penetrance of a phenotype in this CGG-repeat size range.
Benefits of NBS for fragile X syndrome
Arguments in favor of NBS for fragile X syndrome include benefits of early detection and possibilities for families to access early intervention programs. A family could avoid a prolonged search to explain the baby’s developmental problems, and plan for better treatments for all in the family who are affected. While full mutation babies with FXS can benefit from early intervention programs that begin in the first year, such as the Early Start Denver Model (ESDM),15 premutation babies who are delayed also benefit from ESDM and other programs, but treatment data is lacking. Important reasons to diagnose babies with the premutation at the time of birth are similar, and include treatment and follow-up for the baby and benefits for family members. Although premutation babies are far less likely to show developmental problems than full mutation babies, some do develop ASD or intellectual disabilities, and/ or seizures; early intervention is important for this group.16 Indeed, preliminary results seem to indicate that several forms of clinical involvement can occur very early in life.
Some family members may struggle with premutation or full mutation problems, and can benefit from treatment. Reproductive counseling for the mother, and other female carriers in the family tree who may present with clinical symptoms related to involvement associated with the premutation, should be considered.5 However, the needs of the extended family members may be significant and extensive, so time and funding for counseling professionals could limit how many individuals in one family tree can be identified through cascade testing. Therefore, screening could overwhelm and saturate genetic counseling resources and comprehensive care infrastructures, important factors to consider in support of the families with an identified newborn.
Finally, in the past few years we entered into a new age of targeted treatments, with a number of clinical trials for pharmacological interventions, such as minocycline, sertraline, mGluR5 antagonists, and GABAA and GABAB agonists, showing benefits and efficacy for those with fragile X syndrome, including young children.17,18 New targeted treatments will continue to be developed in the future, and multiple family members who are identified through newborn diagnosis and familial cascade testing, and present with clinical involvement, will also benefit from early intervention and long-term follow-up.
Conclusions
Knowledge regarding prevalence is important to guide health policy decisions regarding the effects of FMR1-associated disorders on public health. Emerging, promising results from new targeted treatments for fragile X syndrome and the feasibility of molecular detection could result in adding FMR1 mutations to existing newborn screening programs in the near future. Ideally, a newborn screening program should include parental education, follow-up, diagnosis, and treatment to guarantee the benefits of screening. Thus, it is imperative that services, resources, and infrastructure for early-childhood developmental interventions be expanded, and counseling and educational needs made available so that treatment of emotional/behavioral/developmental problems can be recommended for identified newborns and their families when needed.
Acknowledgments
This work was supported by the NICHD HD02274. Thanks to Dr. Randi J Hagerman for the helpful comments. This work is dedicated to the memory of Matteo.
Dr. Flora Tassone has consulted with Novartis and Genentech.
Footnotes
Conflicts of Interest: None
References
- 1.Martin SB, Huntsman MM. Pathological Plasticity in Fragile X Syndrome. Neural Plasticity. doi: 10.1155/2012/275630. Epub 2012 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey DB, Jr, Raspa M, Bishop E, Holiday D. No change in the age of diagnosis for fragile x syndrome: findings from a national parent survey. Pediatrics. 2009;124(2):527–533. doi: 10.1542/peds.2008-2992. [DOI] [PubMed] [Google Scholar]
- 3.Newborn screening: toward a uniform screening panel and system. Genet Med. 2006;8(Suppl 1)(5):1S–252S. doi: 10.1097/01.gim.0000223891.82390.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagerman RJ, Hagerman PJ, editors. Fragile X Syndrome: Diagnosis, Treatment, and Research. 3rd ed. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- 5.Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12(8):786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Tassone F, Berman RF, et al. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19(1):196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman GE, Le WW, Entezam A, et al. Ovarian abnormalities in a mouse model of fragile X primary ovarian insufficiency. J Histochem Cytochem. 2012;60(6):439–456. doi: 10.1369/0022155412441002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan ES, Cao Z, Hulsizer S, et al. Early mitochondrial abnormalities in hippocampal neurons cultured from Fmr1 pre-mutation mouse model. J Neurochem. 2012;123(4):613–621. doi: 10.1111/j.1471-4159.2012.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Z, Hulsizer S, Cui Y, et al. Enhanced Asynchronous Ca2+ Oscillations Associated with Impaired Glutamate Transport In Cortical Astrocytes Expressing Fmr1 Premutation Expansion. J Biol Chem. 2013;288(19):13831–13841. doi: 10.1074/jbc.M112.441055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tassone F, Iong KP, Tong TH, et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012;4(12):100. doi: 10.1186/gm401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maenner MJ, Baker MW, Broman KW, et al. FMR1 CGG expansions: Prevalence and sex ratios. Am J Med Genet B Neuropsychiatr Genet. 2013;162(5):466–473. doi: 10.1002/ajmg.b.32176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey DB, Wheeler A, Hoffend C, et al. Paper presented at: 1st International Conference on FMR1 Premutation: Basic Mechanisms and Clinical Involvement. Perugia, Italy: 2013. Early development and behavior of FMR1 premutation infants identified by newborn screening. [Google Scholar]
- 13.Rivera S. Paper presented at, 1st International Conference on FMR1 Premutation: Basic Mechanisms and Clinical Involvement. Perugia, Italy: 2013. Jun 23–26, Visual motion processing deficits in infants with the fragile X permutation. [Google Scholar]
- 14.Christie L, Wotton T, Bennetts B, et al. Maternal attitudes to newborn screening for fragile X syndrome. Am J Med Genet A. 2013;161A(2):301–311. doi: 10.1002/ajmg.a.35752. [DOI] [PubMed] [Google Scholar]
- 15.Dawson G, Rogers S, Munson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125(1):e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey DB, Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A(16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 17.Gürkan CK, Hagerman RJ. Targeted treatments in autism and fragile X syndrome. Res Autism Spectr Disord. 2012 Oct 1;(4):1311–1320. doi: 10.1016/j.rasd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winarni TI, Chonchaiya W, Adams E, Au J, Mu Y, Rivera SM, Nguyen DV, Hagerman RJ. Sertraline may improve language developmental trajectory in young children with fragile x syndrome: a retrospective chart review. Autism Res Treat. doi: 10.1155/2012/104317. Epub 2012 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]