Figure 2. Workflow for the discovery, validation, and implementation of new biomarkers.
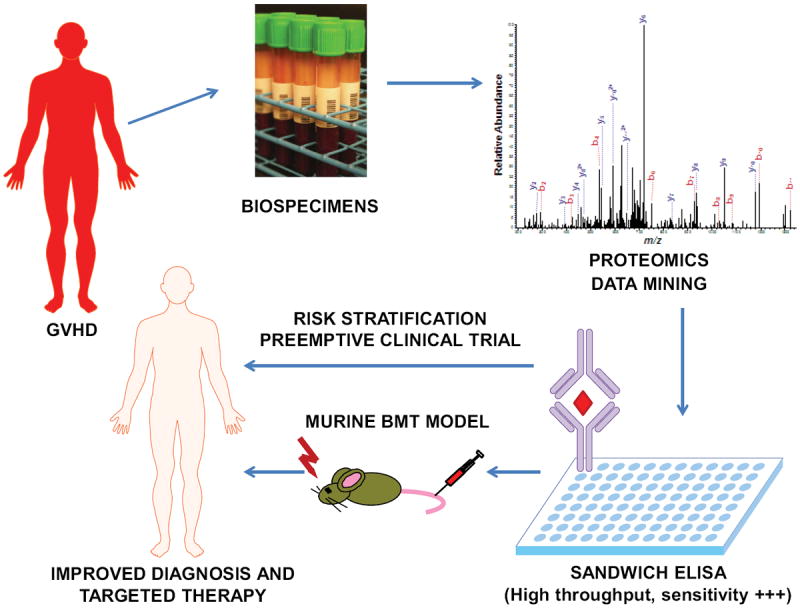
Samples are obtained from patients diagnosed in the clinic with GVHD. The proteins in the sample are subjected to separation and purification subsequently followed by mass spectrometry for protein identification. The protein concentrations from the patients’ samples are then compared to known concentrations of the identified protein in an immunoassay (usually sandwich ELISA). Once a biomarker is validated, it is carried into clinical trials for analysis of its ability as a diagnostic and prognostic tool. The end goal is more personalized treatment and improved patient outcomes.
