Abstract
Objective
To explore potential predictors of self-reported paretic arm use at baseline and after task-specific training (TST) in survivors of stroke.
Design
Data were obtained from a randomized controlled trial of somatosensory stimulation and upper limb TST in chronic stroke.
Setting
University laboratory.
Participants
Chronic (≥3mo) survivors of stroke (N=33; mean age, 62y; mean stroke duration, 38mo).
Interventions
Participants received 12 sessions of TST preceded by either active (n=16) or sham (n=17) somatosensory stimulation to all 3 peripheral nerves.
Main Outcome Measures
Demographic and clinical characteristics were entered stepwise into multiple linear regression analyses to determine the factors that best predict baseline Motor Activity Log (MAL) amount of use rating and change 3 months after TST.
Results
The Action Research Arm Test (ARAT) score predicted the amount of use at baseline (R2=.47, P<.001); in using this model, an ARAT score of 54 (maximum of 57) is required to score 2.5 on the MAL (use described as between rarely and sometimes). After TST the change in the ARAT score predicted the change in the amount of use (R2=.31, P=.001). The predictive power of the model for change at 3 months increased if the Fugl-Meyer Assessment wrist component score was added (R2=.41, P=.001).
Conclusions
Utilization of the paretic upper limb in activities of daily living requires high functional ability. The increase in self-reported arm use after TST is dependent on the change in functional ability. These results provide further guidance for rehabilitation decisions.
Keywords: Rehabilitation, Stroke, Upper extremity
List of abbreviations: ARAT, Action Research Arm Test; CIMT, constraint-induced movement therapy; FMA, Fugl-Meyer Assessment; MAL, Motor Activity Log; MAS, Modified Ashworth Scale; RCT, randomized controlled trial; TST, task-specific training
Restoring motor function after stroke is an important factor for increasing independence in daily activities.1, 2 A challenge in rehabilitation is identifying the main determinants of functional ability and the potential for recovery. This is of paramount importance to guide the planning of therapy goals, to manage the expectations of patients and their cargivers, and for organization of rehabilitation services. The potential for clinical, neurophysiological, and imaging measures to predict the ability to make meaningful recovery has recently received considerable interest.3, 4 However, for chronic brain injury, the relation between motor function and amount of paretic arm use is largely unknown. Previous studies examining change in arm use after constraint-induced movement therapy (CIMT) have found distal arm function to be a significant factor,5, 6 but further investigation of baseline paretic arm use and change after therapy is needed. Whether the arm affected by stroke was previously dominant or nondominant may impact on recovery,7 learned disuse, and the perseverance of survivors of stroke to reintroduce the paretic arm into activities of daily living. Recent evidence suggests that functional ability must be quite high in order for survivors of stroke to regularly use their affected arm,8, 9 and there is a call for further investigation into this.9
Task-specific training (TST) is a rehabilitation technique that involves goal-directed practice of motor tasks with the aim of improving task performance. Patients repeatedly perform functional tasks and are given feedback on their performance.10 TST has been shown to be effective at improving upper limb function after stroke and is regularly used by therapists.10, 11, 12 Improvements in self-reported amount of arm use after TST have been demonstrated,11 but it is unclear what characteristics predict the change in the amount of paretic arm use after a TST intervention.
The aims of this study were to explore, in survivors of chronic stroke, the potential predictors of self-reported amount of arm use (Motor Activity Log [MAL]13) and the potential for increases in the amount of use after TST. We also aimed to determine whether predictors of arm use differed between patients whose dominant and nondominant arms were affected.
Methods
Data for this study were collected during a randomized controlled trial (RCT) of somatosensory stimulation and upper limb TST in survivors of chronic stroke. This was approved by the National Research Ethics Service and registered as an RCT (ISCRTN 05542931). Written informed consent was obtained from each participant. After baseline assessments, participants were block-randomized to receive 2 hours of either active or sham somatosensory stimulation followed by 30 minutes of TST, 3 times per week for 4 weeks. Participants and the assessor (M.K.F.) were blinded to group allocation, but the treating physiotherapist (S.F.R.L.) was not. Two baseline assessments were conducted to ensure stability, and follow-up assessments were conducted immediately after the intervention and at 3 and 6 months. We report the data from the baseline assessments and the 3 month follow-up because there were no differences between groups in any assessment at these time points, and it was thought that 3 months after TST would give a better indication of training-related changes in habitual arm use than immediately after the intervention.
Participants
Participants were recruited for the RCT from local National Health Service sites, stroke support groups, and word of mouth. Inclusion criteria were age >18 years, single stroke of ≥3 months duration, unilateral upper limb weakness, completed upper limb rehabilitation, and the presence of motor-evoked potentials in response to transcranial magnetic stimulation with the muscles either at rest or preactivated (to ensure potential for functional improvement14). Exclusion criteria were contraindications to transcranial magnetic stimulation (eg, epilepsy or seizures), cardiac pacemakers or metal implants in the head, severe spasticity (≥4 on the Modified Ashworth Scale [MAS]15), wheelchair-bound, or presence of dysphasia or cognitive dysfunction sufficient to limit the ability to provide informed consent.
Task-specific training
All participants received 12 sessions (4wk) of TST with an experienced neurophysiotherapist (S.F.R.L.). Each 30-minute session was divided into 6 sections of 5 minutes: stretching and warm-up, grasp, grip, pinch, gross movements, and patient choice. The tasks were based around those required for the Action Research Arm Test (ARAT)16 and were practiced in a pseudo-randomized order in each session.10
Clinical variables (predictors)
Demographic and clinical variables were chosen that are commonly assessed in survivors of stroke in clinical and/or research settings and could be logically thought to have a potential influence on the amount of paretic arm use. Data were obtained from the assessments of the RCT. These variables included age, time since stroke (chronicity), Barthel Index,17 MAS,15 baseline ARAT,18 baseline upper limb Fugl-Meyer Assessment (FMA),19 and change in ARAT and FMA 3 months after TST. The ARAT and FMA are standardized measures of upper limb function.16, 18, 19 The ARAT is formed of 4 subsections: grasp, grip, pinch, and gross. Each task is scored out of 3 (high score means good function, maximum of 57). The FMA is formed of 4 subsections: shoulder, wrist, hand, and coordination. Each task is scored out of 2 (high score means good function, maximum of 66). The subsection scores were also included as potential predictors.
Outcome measure (dependent variable)
The dependent variables were the average baseline MAL amount of use and the change 3 months after TST. The MAL requires participants to report how much (amount of use) they use their affected arm for a selection of daily activities. Ratings are from 0 (arm not used at all) to 5 (used as much as before the stroke).
Data analysis
After confirmation that the 2 baseline assessments were not statistically different (paired t tests), mean values were used for the ARAT, FMA, and MAL. Spearman correlations were performed to determine whether clinical and demographic factors (table 1) correlated with baseline MAL amount of use rating.
Table 1.
Participant characteristics and assessment scores
| Participant | Age (y) | Chronicity (mo) | BI | Affected Arm | MAS | Baseline |
After Physiotherapy |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ARAT | FMA | MAL | Δ ARAT | Δ FMA | Δ MAL | ||||||
| 1 | 82 | 26 | 16 | D | 0 | 40.5 | 52.5 | 2.1 | NA | NA | NA |
| 2 | 78 | 17 | 17 | N | 0 | 46.5 | 58.5 | 2.1 | 5.5 | 3.5 | 0.3 |
| 3 | 70 | 46 | 19 | D | 3 | 28.5 | 34.0 | 0.5 | 1.5 | 2.0 | 0.2 |
| 4 | 84 | 126 | 17 | D | 3 | 10.5 | 25.0 | 0.0 | 3.5 | 2.0 | 0.1 |
| 5 | 63 | 26 | 20 | D | 1 | 42.5 | 60.5 | 1.5 | 2.5 | −1.5 | 0.7 |
| 6 | 54 | 18 | 17 | D | 3 | 10.0 | 24.0 | 0.6 | 2.0 | 1.0 | 0.2 |
| 7 | 77 | 57 | 18 | N | 2 | 19.5 | 33.5 | 0.4 | 0.5 | −3.5 | 0.6 |
| 8 | 62 | 76 | 16 | N | 1 | 37.5 | 42.0 | 0.7 | 2.5 | 2.0 | 0.3 |
| 9 | 50 | 60 | 20 | D | 0 | 31.5 | 57.5 | 2.4 | 5.5 | −1.5 | 0.7 |
| 10 | 74 | 3 | 14 | N | 0 | 43.5 | 51.0 | 1.4 | 7.5 | 8.0 | 1.4 |
| 11 | 64 | 89 | 20 | D | 1 | 37.5 | 42.0 | 2.9 | 4.5 | 5.0 | 0.0 |
| 12 | 74 | 53 | 20 | D | 1 | 29.5 | 52.0 | 1.8 | 9.5 | 5.0 | 0.7 |
| 13 | 35 | 14 | 18 | N | 3 | 30.0 | 43.0 | 1.7 | 4.0 | 4.0 | −0.7 |
| 14 | 77 | 37 | 15 | N | 3 | 10.5 | 22.0 | 0.4 | 0.5 | 0.0 | −0.1 |
| 15 | 79 | 18 | 17 | N | 2 | 36.5 | 33.0 | 1.4 | NA | NA | NA |
| 16 | 49 | 16 | 16 | D | 0 | 29.5 | 43.0 | 0.9 | 7.5 | 5.0 | 1.1 |
| 17 | 45 | 16 | 19 | D | 1 | 37.0 | 44.5 | 2.4 | 2.0 | 2.5 | 0.1 |
| 18 | 55 | 4 | 20 | N | 0 | 48.5 | 47.0 | 1.3 | 2.5 | 4.0 | 1.2 |
| 19 | 24 | 124 | 20 | D | 2 | 11.0 | 31.0 | 0.4 | 5.0 | 3.0 | −0.1 |
| 20 | 61 | 58 | 17 | N | 1 | 37.0 | 39.0 | 1.9 | 3.0 | 11.0 | 0.2 |
| 21 | 57 | 7 | 17 | D | 0 | 38.0 | 38.0 | 2.4 | −3.0 | −2.0 | −0.5 |
| 22 | 66 | 6 | 20 | N | 2 | 35.5 | 34.0 | 2.0 | −4.5 | 4.0 | −0.2 |
| 23 | 45 | 3 | 18 | N | 1 | 35.5 | 45.0 | 1.9 | 9.5 | 9.0 | 2.0 |
| 24 | 65 | 54 | 19 | D | 3 | 9.0 | 26.5 | 0.4 | −1.0 | −0.5 | 0.6 |
| 25 | 69 | 54 | 20 | D | 2 | 10.0 | 34.0 | 1.5 | NA | NA | NA |
| 26 | 59 | 4 | 20 | N | 0 | 38.0 | 46.0 | 1.5 | −10.0 | −12.0 | −0.9 |
| 27 | 54 | 17 | 18 | D | 0 | 38.5 | 48.0 | 2.7 | 1.5 | 6.0 | 1.2 |
| 28 | 60 | 8 | 20 | D | 1 | 34.5 | 30.0 | 1.9 | 4.5 | 9.0 | 1.0 |
| 29 | 64 | 130 | 20 | D | 2 | 34.5 | 54.5 | 1.6 | 0.5 | −2.5 | 1.6 |
| 30 | 56 | 13 | 19 | D | 2 | 29.0 | 37.5 | 1.6 | −1.0 | 1.5 | 0.2 |
| 31 | 36 | 43 | 20 | N | 1 | 16.0 | 30.0 | 1.0 | 1.0 | −1.0 | 0.0 |
| 32 | 66 | 9 | 16 | D | 1 | 20.0 | 33.5 | 0.7 | 2.0 | 3.5 | 0.0 |
| 33 | 74 | 12 | 17 | N | 1 | 18.0 | 29.5 | 0.8 | 4.0 | 1.5 | 0.0 |
| Mean ± SD | 61.5±14.2 | 37.7±36.7 | 18.2±1.8 | NA | 1.3±1.1 | 29.5±11.9 | 40.0±10.5 | 1.4±.8 | 2.4±4.0 | 2.3±4.5 | 0.4±0.7 |
| Minimum | 24.0 | 3.0 | 14.0 | NA | 0.0 | 9.0 | 22.0 | 0.0 | −10.0 | −12.0 | −0.9 |
| Maximum | 84.0 | 130.0 | 20.0 | NA | 3.0 | 48.5 | 60.5 | 2.9 | 9.5 | 11.0 | 2.0 |
Abbreviations: BI, Barthel Index; Δ, change; D, dominant; N, nondominant; NA, not applicable.
Forward stepwise multiple linear regression analysesa were conducted to explore the variables that predicted baseline MAL amount of use and change in the amount of use 3 months after TST. Because this study was exploratory, all clinical variables were entered as potential predictors. The criterion for keeping a variable in the forward stepwise regression was a significant contribution to the model (P≤.05). The criterion for removing a variable was if it was not making a significant contribution to the model (P≥0.1). Paired t tests were used to compare the ARAT, FMA, and MAL scores before and after TST. Significance was set at alpha=.05.
Results
Thirty-three patients (13 women; mean age, 61.5y) were included. Participant characteristics and assessment scores are presented in table 1. There were no significant differences in function or MAL scores between those who received active (n=16) or sham (n=17) somatosensory stimulation at baseline or for the changes 3 months after TST (independent samples t test; P>.05); therefore, all participants were grouped together for the analyses. The mean time since stroke ± SD was 37.7±36.7 months, baseline ARAT score was 29.5±11.9, and FMA score was 40.0±10.5. All participants were right handed prior to stroke, and 19 had their right arm affected. Three participants failed to attend the 3-month follow-up assessment; therefore, their data are not included for the prediction of change in MAL amount of use.
Correlations with the amount of use
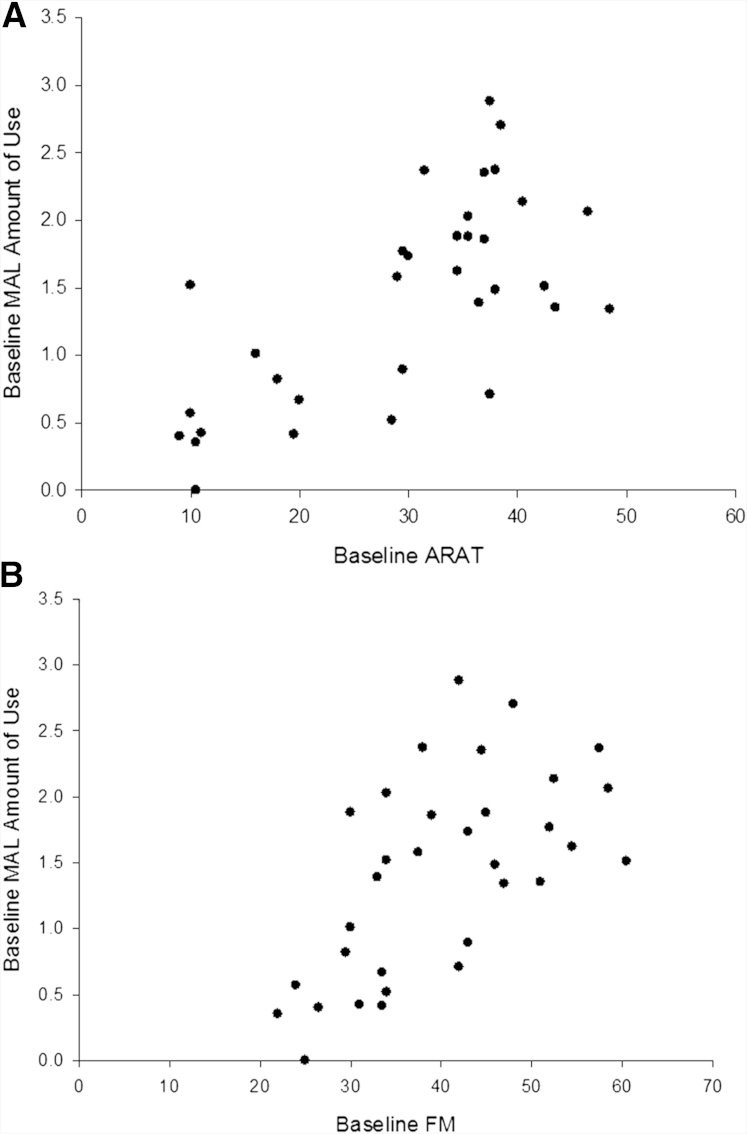
The results of the Spearman correlations are presented in table 2. There was a significant negative correlation between the amount of use and the MAS (P=.001), and there were positive correlations with the ARAT and FMA (P<.01) (fig 1).
Table 2.
Spearman correlations with baseline amount of use rating (MAL)
| Independent Variable | R | P |
|---|---|---|
| Chronicity | −.274 | .123 |
| MAS | −.565 | .001∗ |
| Age | −.137 | .446 |
| Barthel Index | .275 | .122 |
| Baseline ARAT | .685 | <.001∗ |
| Baseline FMA | .611 | <.001∗ |
| ARAT subcomponents | ||
| Grasp | .670 | <.001∗ |
| Grip | .645 | <.001∗ |
| Pinch | .609 | <.001∗ |
| Gross | .537 | <.001∗ |
| FMA subcomponents | ||
| Shoulder | .546 | .001∗ |
| Wrist | .489 | .004∗ |
| Hand | .504 | .003∗ |
| Coordination | .080 | .656 |
Abbreviation: R, correlation coefficient.
P<.05.
Fig 1.
Scatterplot showing the relation between the ARAT score (A), FMA score (B), and MAL amount of use rating at baseline.
Predicting baseline amount of use
The baseline ARAT score predicted 47% of the variability in baseline MAL amount of use (F1,31=27.457; P<.001). In using the equation for the regression model, an ARAT score of 54 is required to reach an amount of use score of 2.5 (half the maximum value, described as between rarely and half as much as before the stroke). All other clinical variables were excluded, not significantly adding to the predictive power of the model (all P>.19).
If participants were examined separately based on which hand was affected, the baseline ARAT score still strongly predicted the amount of use for those with the dominant hand affected (R2=0.6; F1,17=25.518; P<.001). The equation for this regression model calculates that an ARAT score of 46 is required for an amount of use score of 2.5.
For participants with the nondominant hand affected, the ARAT gross component score predicted 56.8% of the variability in the amount of use (F1,12=15.806; P=.002). The equation for the regression model calculates that patients will not score ≥2.5 even if they reach a maximum score on the grasp component of the ARAT. The predictive power of the model was further increased when the FMA wrist component score was added (R2=0.7; F2,11=13.069; P=.001).
Predicting change in the amount of use after TST
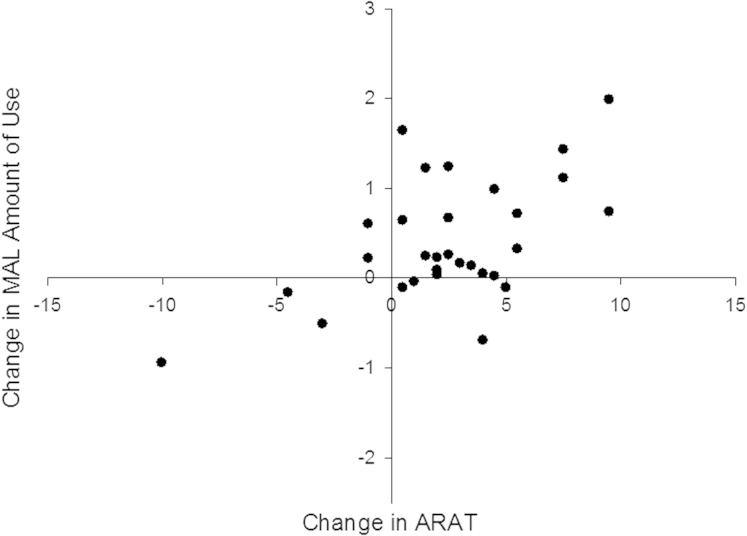
ARAT, FMA, and MAL scores increased significantly after TST (P<.01) (see table 1). Changes in the ARAT score predicted 30.8% of the variability in change in MAL amount of use (F1,28=12.486; P=.001). The relation between change in ARAT score and change in the amount of use is presented in figure 2. The predictive power of the model increased to 41.1% when the FMA wrist component score at baseline was added (F2,27=9.424; P=.001). All other clinical variables were excluded because they did not significantly add to the predictive power of the model (all P>.11).
Fig 2.
Scatterplot showing the relation between the change in the ARAT score and the change in the MAL amount of use rating after TST.
For the participants with the dominant hand affected, the baseline FMA wrist score predicted 30.6% of the variability in change in the amount of use (F1,15=6.601; P=.021). For participants with the nondominant hand affected, the change in the grasp component of the ARAT predicted 58.8% of change in the amount of use (F1,11=15.674; P=.002).
Discussion
This exploratory study describes the relation between functional ability and self-reported amount of paretic arm use in survivors of chronic stroke (≥3mo) at baseline and after 4 weeks of TST. Although most participants were fairly independent (average Barthel Index score of 18.2), the paretic arm was reportedly used for daily activities for less than half of the time. Upper limb function predicted the amount of use rating at baseline, and the change in function predicted the change in the amount of use rating after TST, indicating that good functional ability is necessary to promote upper limb utilization.
Both the ARAT and FMA were found to correlate positively with the baseline MAL score, confirming previous findings8, 20, 21; spasticity (MAS) was negatively correlated with the amount of use. However, 31 of the 33 participants scored <2.5 on the MAL, indicating that they use their paretic hand substantially less than prior to stroke. Participants with an ARAT score ≤20 or a FMA score ≤30 had average MAL scores of 0.6 and 0.7 respectively, indicating virtually no use of the paretic limb (see fig 1). For the participants who scored the maximum of 20 on the Barthel Index (indicating full independence), the average MAL score was 1.6, providing further support that global measures lack sensitivity and, therefore, may be unsuitable to capture the effects of therapy in clinical trials.9 The regression model indicated that an ARAT score ≥54 (out of 57) would be necessary before the amount of use rating would exceed 2.5 (between rarely and half as much as before the stroke). This suggests that the functional ability of the upper limb needs to be almost perfect before patients will begin to habitually engage the arm in daily activities.
When the dominant hand was affected by stroke, the predictive power of the ARAT score was higher than when all patients were grouped together, and the ARAT score necessary to achieve an MAL score of 2.5 was reduced to 46. This indicates that survivors of stroke are more likely to use their affected hand, even in the presence of more severe paresis, if they habitually used it for most activities prior to the stroke. This may suggest that learned disuse is easier to overcome if the dominant hand is affected. However, in patients whose stroke affected their nondominant hand, the model predicted that even perfect gross upper limb function would not be sufficient to ensure that they would use their upper limb regularly. If confirmed by other studies, particularly with objective measures of arm use, this could have serious implications for therapy decisions.
All of the participants had the potential to achieve meaningful improvements in function with training14; after 4 weeks of TST, functional ability and amount of use rating increased significantly. Change in the ARAT score was found to predict 30.8% of the change in MAL amount of use, further supporting the idea that functional improvement is necessary for increased arm use. The predictive model was strengthened by the inclusion of the baseline FMA wrist subcomponent score, indicating that the ability to make movements at the wrist is an important factor for making gains in arm use after therapy. This is a stronger model than those reported previously after CIMT5, 6 and confirms that prioritizing physical therapy for survivors of stroke with some degree of distal hand function could enhance the possibility of making gains in paretic arm use. This may be particularly so for participants with the dominant hand affected, in which the baseline FMA wrist score was found to be the main predictor of change in the amount of use.
Study limitations
This study has explored potential predictors of self-reported paretic arm use rather than actual arm use. Although the MAL has been found to be reliable and valid,13 it is a subjective measure rather than an objective one. One advantage of a self-report measure over those from other devices (eg, accelerometers) includes the ability to capture the stroke survivor's perspective of how his or her arm use has changed. Even if not truly reflective of actual arm use, his or her opinion is important. However, results could be affected by a participant's desire to please the investigator or poor recall of actual use. The perspective of the survivor of stroke is increasingly considered as an important way to measure the impact of stroke and outcomes after rehabilitation.
One limitation is that all participants were in the chronic phase of stroke recovery (range, 3–130mo) and had completed and been discharged from standard upper limb rehabilitation. It remains to be determined whether the predictors of change in MAL score will be the same in the early period after brain injury when more spontaneous recovery will occur. Interestingly, time since stroke did not correlate with the baseline MAL score or predict either the baseline MAL score or change after TST. In addition, the regression model explains a small-to-moderate proportion of the variance in self-reported arm use; therefore, there are other possible factors that may determine a patient's perception of his or her arm use. It is important to continue to investigate other possible determinants to help guide rehabilitation goals. These could include aspects such as living situation, cognitive status, work, or other activity requirements. These aspects were not assessed as part of this study but could potentially contribute significantly. One of the strengths of this study is that patients had a range of ages and stroke durations; neither of these factors appeared to influence the amount of use or the potential to increase the amount of use with TST. However, this is in contrast with Lin,6 Fritz,22 and colleagues, who reported age to be a predictor of change in the amount of use after CIMT. The differences may lie in the types of therapy delivered. CIMT is an intense rehabilitation regimen requiring restraint of the unaffected upper limb and making it essential for patients to use their paretic arm for activities. In contrast, TST (as used in the present study) involved less intense retraining of the paretic limb without specifically inhibiting use of the less affected arm. It is conceivable that age may affect the response to the 2 therapy interventions differently, and this could impact on behavioral change, which has clinical implications for therapeutic provision.
Conclusions
There is a substantial amount of research underway to attempt to predict the chance of recovery of arm function after stroke. These results provide further information to guide rehabilitation decisions, providing support for the idea that high functional ability is important for survivors of stroke to report adequate use of the upper limb in activities of daily living.
Supplier
-
a.
SPSS; IBM UK Ltd, PO Box 41, North Harbour, Portsmouth, Hampshire PO6 3AU, England.
Acknowledgments
We thank Tony Christopher and Lindsey Marjoram, BSc, for technical help.
Footnotes
Supported by The Dunhill Medical Trust (grant no. R102/0209) and the South London Clinical Local Research Network.
Clinical Trial Registration No.: ISRCTN 05542931.
No commercial party having a direct financial interest in the results of the research supporting this article has conferred or will confer a benefit on the authors or on any organization with which the authors are associated.
References
- 1.Schiemanck S.K., Kwakkel G., Post M.W., Kappelle L.J., Prevo A.J. Predicting long-term independency in activities of daily living after middle cerebral artery stroke: does information from MRI have added predictive value compared with clinical information? Stroke. 2006;37:1050–1054. doi: 10.1161/01.STR.0000206462.09410.6f. [DOI] [PubMed] [Google Scholar]
- 2.Veerbeek J.M., Kwakkel G., van Wegen E.E., Ket J.C., Heymans M.W. Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke. 2011;42:1482–1488. doi: 10.1161/STROKEAHA.110.604090. [DOI] [PubMed] [Google Scholar]
- 3.Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–1232. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]
- 4.Stinear C.M., Ward N.S. How useful is imaging in predicting outcomes in stroke rehabilitation? Int J Stroke. 2013;8:33–37. doi: 10.1111/j.1747-4949.2012.00970.x. [DOI] [PubMed] [Google Scholar]
- 5.Li K.Y., Lin K.C., Wang T.N., Wu C.Y., Huang Y.H., Ouyang P. Ability of three motor measures to predict functional outcomes reported by stroke patients after rehabilitation. NeuroRehabilitation. 2012;30:267–275. doi: 10.3233/NRE-2012-0755. [DOI] [PubMed] [Google Scholar]
- 6.Lin K.C., Huang Y.H., Hsieh Y.W., Wu C.Y. Potential predictors of motor and functional outcomes after distributed constraint-induced therapy for patients with stroke. Neurorehabil Neural Repair. 2009;23:336–342. doi: 10.1177/1545968308321773. [DOI] [PubMed] [Google Scholar]
- 7.Darling W.G., Helle N., Pizzimenti M.A. Laterality affects spontaneous recovery of contralateral hand motor function following motor cortex injury in rhesus monkeys. Exp Brain Res. 2013;228:9–24. doi: 10.1007/s00221-013-3533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart J.C., Cramer S.C. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44:1111–1116. doi: 10.1161/STROKEAHA.111.674671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stinear C.M., Byblow W.D. Letter by Stinear and Byblow regarding article, “patient-reported measures provide unique insights into motor function after stroke”. Stroke. 2013;44:e79. doi: 10.1161/STROKEAHA.113.001689. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard I.J., Parsons M.W., Neilson C., Carey L.M. Task-specific training: evidence for and translation to clinical practice. Occup Ther Int. 2009;16:175–189. doi: 10.1002/oti.275. [DOI] [PubMed] [Google Scholar]
- 11.Arya K.N., Verma R., Garg R.K., Sharma V.P., Agarwal M., Aggarwal G.G. Meaningful task-specific training (MTST) for stroke rehabilitation: a randomized controlled trial. Top Stroke Rehabil. 2012;19:193–211. doi: 10.1310/tsr1903-193. [DOI] [PubMed] [Google Scholar]
- 12.Winstein C.J., Rose D.K., Tan S.M., Lewthwaite R., Chui H.C., Azen S.P. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: a pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil. 2004;85:620–628. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Uswatte G., Taub E., Morris D., Light K., Thompson P.A. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 14.Stinear C.M., Barber P.A., Smale P.R., Coxon J.P., Fleming M.K., Byblow W.D. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 15.Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540–542. [PubMed] [Google Scholar]
- 16.Yozbatiran N., Der-Yeghiaian L., Cramer S.C. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
- 17.Collin C., Wade D.T., Davies S., Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10:61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 18.Lyle R.C. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Fugl-Meyer A.R., Jaasko L., Leyman I., Olsson S., Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 20.van der Lee J.H., Beckerman H., Knol D.L., de Vet H.C., Bouter L.M. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35:1410–1414. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 21.Pereira N.D., Ovando A.C., Michaelsen S.M. Motor Activity Log-Brazil: reliability and relationships with motor impairments in individuals with chronic stroke. Arq Neuropsiquiatr. 2012;70:196–201. doi: 10.1590/s0004-282x2012000300008. [DOI] [PubMed] [Google Scholar]
- 22.Fritz S.L., Light K.E., Clifford S.N., Patterson T.S., Behrman A.L., Davis S.B. Descriptive characteristics as potential predictors of outcomes following constraint-induced movement therapy for people after stroke. Phys Ther. 2006;86:825–832. [PubMed] [Google Scholar]