Abstract
Low and high birth weights have been linked to increased susceptibility to cardiovascular and metabolic alterations. However, the natural history of cardiometabolic disturbances in children born small (SGA) and large (LGA) for gestational age is still unclear and no reliable biomarker of cardiovascular risk has definitively been identified in these subjects. Interestingly, asymmetric dimethylarginine (ADMA), antagonist of nitric oxide (NO) production, has been recognized as novel cardiovascular marker able to identify subjects at higher risk of health disturbances. Despite the well-described role of ADMA as a predictor of degenerative disease in adults, its potential application in pediatrics, and specifically in SGA and LGA children, has not been explored as only few data in preterm infants and SGA newborns are available. Therefore, we investigated potential alterations in circulating ADMA and NO levels in SGA and LGA children compared with those born appropriate (AGA) for gestational age. Of note, ADMA was significantly higher in SGA and LGA children than AGA peers. Intriguingly, SGA and LGA categories as well as insulin resistance were independently related to ADMA. Our observations lead to the intriguing hypothesis that ADMA could be involved in the development of cardiometabolic alterations in SGA and LGA children already during the prepubertal age. Antioxid. Redox Signal. 20, 2317–2322.
Size at Birth and Future Cardiovascular and Metabolic Risk
There is accumulating evidence supporting a strong link between the size at birth and adult susceptibility to cardiovascular and metabolic diseases (5, 7). As a matter of fact, many studies have focused on the cardiometabolic profile of subjects born small (SGA) and large (LGA) for gestational age, revealing an increased risk of a wide range of chronic disorders and metabolic abnormalities, including insulin resistance (IR), alterations of the oxidant/antioxidant status, obesity, disturbances of glucose metabolism, abnormal blood pressure, and coronary heart disease (5, 7). Of great concern, many of these disorders have been detected in SGA and LGA subjects not only during adulthood, but also during childhood (3), raising the issue that variations in the fetal size may have crucial implications for health even at a young age. Among all, the development of IR represents the most common and early alteration in children born SGA and LGA, predisposing them to possible health disturbances (3). These observations are strongly supported by our results. In fact, in the present study, fasting insulin (FI) and homeostasis model assessment of IR (HOMA-IR) were significantly higher in SGA children when compared with appropriate for gestational age (AGA) peers (Table 1). Similarly, LGA children showed higher FI and HOMA-IR values when compared with the AGA group (Table 1). These findings confirm an impairment of insulin sensitivity in SGA and LGA subjects already during the prepubertal age. Unfortunately, no straightforward and definite explanation is available for the reduced insulin sensitivity in these birth weight (BW) categories. Nevertheless, an abnormal intrauterine milieu and the growth pattern during infancy seem to exert a profound influence on the development of IR and the related unfavorable outcomes. In fact, it has been found that children born SGA and LGA seem to be prone to increased oxidative stress already during infancy, most likely because of the reduced insulin sensitivity (3). Based on these findings, more effort should be made to clarify the natural history of cardiometabolic disturbances in children born SGA and LGA. In addition, the identification of novel and reliable biomarkers of cardiovascular risk would be extremely helpful to identify SGA and LGA subjects with an adverse metabolic profile.
Table 1.
Clinical and Metabolic Characteristics of the Study Population
| AGA | SGA | LGA | pa | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Number | 32 | 27 | 24 | |
| Age (year) | 7.5±1.6 | 6.7±1.8 | 7.0±2.0 | NS |
| Sex (M/F) | 11M/21F | 13M/14F | 9M/15F | 0.54b |
| Birth weight (g) | 3359±257 | 2509±101 | 4174±258 | <0.001c |
| Height SDS | 0.5±1.2 | 0.3±1.0 | 0.8±1.3 | NS |
| BMI (kg/m2) | 22.7±5.2 | 20.1±5.2 | 23.1±5.7 | NS |
| BMI SDS | 1.3±1.1 | 0.8±1.0 | 1.4±1.1 | NS |
| SBP (mmHg) | 99±8 | 100±6 | 100±8 | NS |
| DBP (mmHg) | 60±6 | 61±5 | 64±6 | NS |
| Lipid profile | ||||
| Total cholesterol (mg/dl) | 159.7±17.0 | 157.9±18.8 | 161.1±17.0 | NS |
| HDL cholesterol (mg/dl) | 57.1±8.5 | 56.3±6.8 | 52.4±5.8 | NS |
| LDL cholesterol (mg/dl) | 88.5±17.6 | 87.4±19.0 | 91.3±15.4 | NS |
| TG (mg/dl)d | 71.9±23.9 | 70.4±19.9 | 83.4±20.0 | NS |
| Insulin resistance | ||||
| Glucose (mg/dl) | 81.6±8.4 | 83.9±7.8 | 85.1±5.6 | NS |
| FI (μU/ml)d | 7.3±3.5 | 10.7±5.2 | 11.4±5.8 | 0.01c |
| HOMA-IRd | 1.51±0.75 | 2.24±1.21 | 2.44±1.31 | 0.008c |
Values are mean±standard deviation. NS, no significant difference (p>0.05).
One-way analysis of variance.
Fisher's exact test.
Significant differences by post hoc analysis.
Values are log transformed.
AGA, appropriate for gestational age; BMI, body mass index; DBP, diastolic blood pressure; F, female; FI, fasting insulin; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; LGA, large for gestational age; M, male; SBP, systolic blood pressure; SDS, standard deviation score; SGA, small for gestational age; TG, triglycerides.
Innovation.
Children born small (SGA) and large (LGA) for gestational age are at increased cardiometabolic risk. Interestingly, asymmetric dimethylarginine (ADMA) has been recognized as a promising biomarker of cardiovascular risk in subjects with chronic disorders; however, no data are available in SGA and LGA youth. This study represents the first evidence of increased ADMA in SGA and LGA children. Intriguingly, SGA and LGA categories were independently related to ADMA. The novelty of this study is that ADMA could represent a fascinating risk marker in SGA and LGA children. Characterization of this biomarker represents a milestone in the recognition of birth weight-related alterations.
Asymmetric Dimethylarginine: A Novel and Fascinating Marker of Cardiometabolic Risk
Interestingly, among the several plasma biomarkers potentially useful in predicting future health disorders, asymmetric dimethylarginine (ADMA) has recently been identified as a novel cardiovascular risk factor and diagnostic marker able to identify subjects at increased risk of cardiometabolic alterations (1). Specifically, ADMA is an endogenous amino acid derived from proteolytic breakdown of arginine-methylated proteins by protein arginine methyltransferases (PRMTs) during the hydrolytic protein turnover (1). The mechanisms of action of ADMA include its competition with l-arginine, the substrate of nitric oxide synthase (NOS), leading to decreased nitric oxide (NO) release by the endothelium. Because NO is involved in maintaining vascular homeostasis, its diminished synthesis determines endothelial dysfunction and vascular inflammation (8). Therefore, increased ADMA levels initiate a cascade of events that, through the antagonization of NO-induced endothelium vasodilation, culminate in the development of vascular dysfunctions, such as vasoconstriction and structure remodeling leading to atherosclerosis (1). ADMA metabolism occurs mainly through its degradation to citrulline and dimethylamine by dimethylarginine dimethylaminohydrolase (DDAH) (1, 8). Therefore, elevations of plasma ADMA concentrations may derive from increased production and/or reduced degradation. Of note, among the potential mechanisms, it has recently been reported that increased oxidative stress and inflammation (2), which characterize many cardiometabolic disorders, are able to interfere with the activity and expression of PRMTs and DDAH, leading to a perturbation of ADMA levels. In addition, high oxidative stress plays a pivotal role in the suppression of NO synthesis by decreasing the expression of NOS gene.
Up to now, extensive data have been accumulated on the presence of increased ADMA concentrations in adults with pathological conditions, such as coronary artery disease, hypercholesterolemia, hypertension, diabetes, and chronic kidney disease, when compared with matched healthy subjects (8). In these disorders, high circulating levels of ADMA have been detected, leading to an increased risk for developing cardiovascular events and heart mortality. Therefore, there is suggestive evidence that ADMA itself may be a mediator of vascular dysfunction besides being a biomarker of cardiometabolic risk (1, 8). A better understanding of the pathophysiology of ADMA and its causal role in the development of several cardiovascular and metabolic disorders would allow potentiating its future clinical application.
Could ADMA Represent a Reliable Biomarker of Cardiometabolic Risk in SGA and LGA Children?
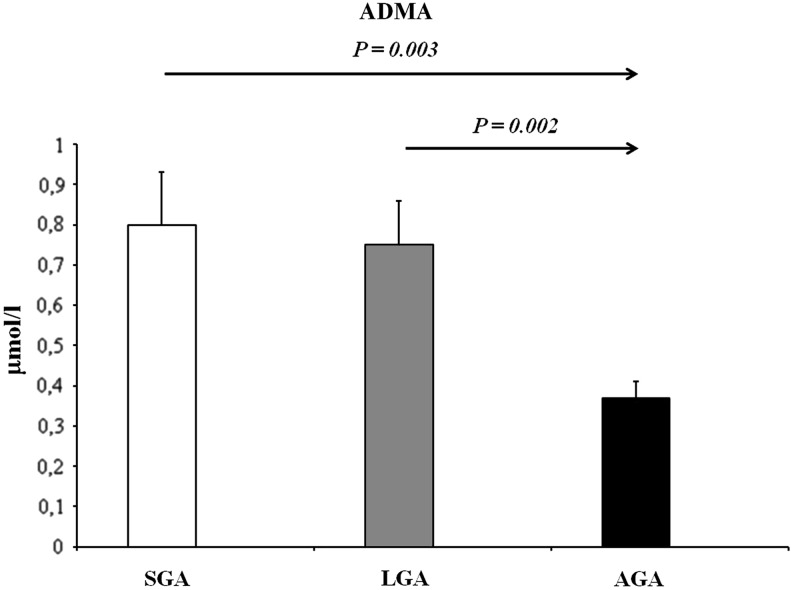
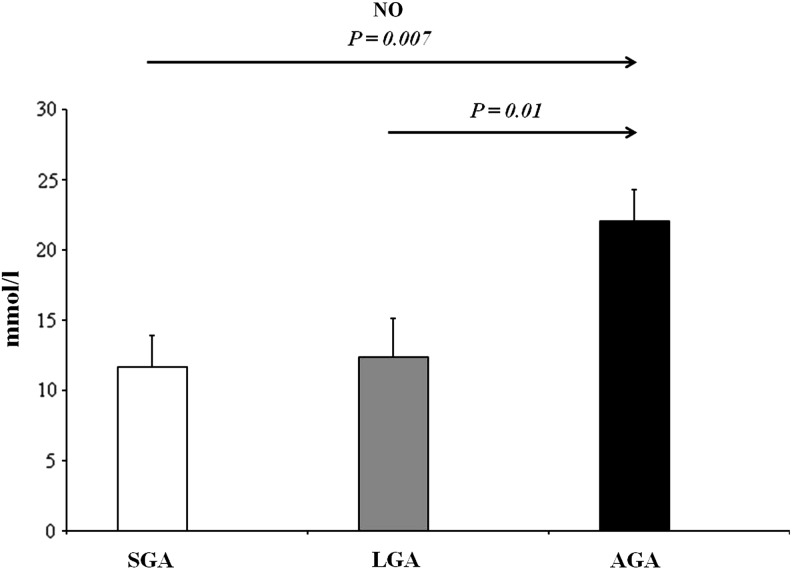
In recent years, a considerable amount of interest has been focused on the potential pathophysiological role of ADMA in the pediatric population. In fact, to date, there is a growing number of studies reporting increased ADMA concentrations in children and adolescents affected by several chronic disorders (8), suggesting a possible reliability of this marker also in childhood. In the pediatric population, pathological conditions with increased ADMA concentrations include hypertension, hypercholesterolemia, congenital heart defects, diabetes, and chronic kidney disease (8). In addition, a growing number of studies indicate a perturbation of the ADMA-NO pathway even in a perinatal setting. Vida et al. detected a progressive increase of ADMA levels in preterm infants during the first month of life, suggesting that early endothelial dysfunction may take part in the developmental programming of chronic adult diseases (9). Nevertheless, up to now, studies assessing ADMA levels have been limited to term and preterm SGA newborns (6), while no data are available in the LGA population. Therefore, to the best of our knowledge, in the present study, we have provided the first evidence of a significant difference in terms of circulating ADMA levels among prepubertal children born SGA and LGA when compared with AGA peers. In fact, in the post hoc analysis, SGA children showed significantly higher ADMA levels than AGA peers (Fig. 1). Similarly, LGA children showed higher ADMA levels when compared with the AGA group (Fig. 1). A significant difference among the three BW categories was also found in terms of NO concentrations. In fact, in the post hoc analysis, NO values were significantly lower in SGA children when compared with AGA subjects (Fig. 2). Accordingly, LGA children showed lower NO values than the AGA group (Fig. 2). These data are supported by a previous study by Franco et al. reporting that urinary NO concentrations were significantly reduced in 8- to 13-year-old SGA children when compared with those born AGA (4). The reasons underlying this significant alteration of the ADMA-NO pathway still remain speculative in SGA and LGA categories. A possibility in this respect is that it might represent the consequence of the reduced insulin sensitivity observed in these risk groups.
FIG. 1.
ADMA concentrations of the study populations. p<0.001, by one-way analysis of variance. Significant values by post hoc analysis. Values are expressed as mean±standard error of mean. ADMA, asymmetric dimethylarginine; AGA, appropriate for gestational age; LGA, large for gestational age; SGA, small for gestational age.
FIG. 2.
NO concentrations of the study populations. p=0.003, by one-way analysis of variance. Significant values by post hoc analysis. Values are expressed as mean±standard error of mean. NO, nitric oxide.
Therefore, based on our report, it could be supposed that ADMA represents a reliable risk marker in SGA and LGA children already during the prepubertal age. In addition, our observations lead to the fascinating hypothesis that a perturbation in the ADMA-NO pathway could be implicated in the cardiometabolic derangements of SGA and LGA children.
Is ADMA Associated with Being Born SGA and LGA?
Based on the finding that ADMA was significantly higher in SGA and LGA children than AGA subjects, we explored the potential association of this marker with BW. Notably, in a multiple linear regression analysis with ADMA as the dependent variable and the BW categories (AGA vs. SGA and AGA vs. LGA) as the independent variables, SGA and LGA categories were significantly and independently related to ADMA (Table 2). The associations between BW categories and ADMA persisted after adjustment for age, sex, and body mass index (BMI) standard deviation score (SDS). These findings could be the result of exposure to an unfavorable environment during the critical period of intrauterine life, contributing to the development of pathophysiological alterations (3). Hence, our observations are consistent with the concept that both low and high BW act significantly as strong independent risk factors for the development of future cardiovascular and metabolic alterations.
Table 2.
Multiple Linear Regression to Evaluate Association Between Asymmetric Dimethylarginine, Birth Weight Categories, and Insulin Resistance
| Dependent variable: ADMA | ||
|---|---|---|
| Independent variable | β coefficient | p |
| Model A (age, sex, BMI SDS) | ||
| BW group: AGA vs. SGA | 0.309 | 0.03 |
| BW group: AGA vs. LGA | 0.319 | 0.02 |
| Insulin | 0.317 | 0.02 |
| Model B (age, sex, BMI SDS) | ||
| BW group: AGA vs. SGA | 0.295 | 0.04 |
| BW group: AGA vs. LGA | 0.296 | 0.04 |
| HOMA-IR | 0.315 | 0.03 |
BW, birth weight.
Of great interest, FI and HOMA-IR were other independent predictors of ADMA (Table 2). These results provide convincing evidence of a significant effect, not only of BW but also of IR on ADMA concentrations, suggesting that the impairment of insulin sensitivity in SGA and LGA children possibly contributes to the development of metabolic disturbances even during infancy. In this respect, increased ADMA concentrations have been observed in adults with diabetes when compared with healthy controls, with a close correlation between IR and ADMA (1, 8). Based on these data, in SGA and LGA categories, we assumed that IR could be involved in the increase of ADMA levels, possibly participating in common signaling pathways. However, the exact relationship between IR and ADMA remains speculative and further studies are needed to elucidate the cause–effect mechanisms underlying this association.
In addition, a multiple linear regression analysis was performed to assess the independent contribution of BW categories on NO concentrations. In this model, with NO as the dependent variable and again BW categories as the independent variables, SGA and LGA categories were associated with NO, independently of age, sex, and BMI SDS (Table 3). These findings are in agreement with results described by Franco et al., who found that NO concentrations correlated significantly with BW before and after adjustment for age and sex. The authors also found that BW and NO were independent determinants affecting vascular function, measured by assessing brachial artery flow-mediated dilation (4). Taken together, these data reinforce the hypothesis of a close link between BW and NO, which is likely involved in the cardiometabolic programming of SGA and LGA children.
Table 3.
Multiple Linear Regression to Evaluate Association Between Nitric Oxide and Birth Weight Categories
| Dependent variable: NO | ||
|---|---|---|
| Independent variable | β coefficient | p |
| Model (age, sex, BMI SDS) | ||
| BW group: AGA vs. SGA | −0.384 | 0.003 |
| BW group: AGA vs. LGA | −0.332 | 0.009 |
NO, nitric oxide.
Limitations of the Study
It might be argued that a major limitation of our study is related to the relatively small sample size as this might implicate that the obtained results are not easily applicable to the whole population of prepubertal SGA and LGA children. However, the presence of three different BW categories extensively investigates a possible alteration of the ADMA-NO pathway. In addition, it needs to be acknowledged that a potential limitation of the present study is its cross-sectional design, which does not allow the demonstration of pathogenetic mechanisms. Moreover, we did not perform a direct assessment of glomerular filtration rate assessment, which may influence circulating ADMA concentrations. Nevertheless, the young age of the study population and the detection of increased ADMA levels suggest that this biomarker may provide helpful information about the presence and severity of cardiovascular and metabolic alterations in SGA and LGA children.
Conclusions and Future Directions
This study represents the first evidence of a perturbation of the ADMA-NO pathway in prepubertal children born SGA and LGA. Of note, both BW and IR seem to play an important role in the induction of increased ADMA levels. Intriguingly, our observations lead to the hypothesis that ADMA could take part in the development of cardiometabolic alterations in SGA and LGA children besides representing a novel and fascinating risk marker of BW-related alterations. New research directions on ADMA are needed to better define the implication of this marker in the development of health disturbances in SGA and LGA categories.
Notes
Study population
We recruited 83 Caucasian prepubertal children, referred to the Department of Pediatrics of the University of Chieti, Italy, after having been admitted for minor diseases. All children were born full term (≥37 weeks of completed gestation) and singleton. In addition, no child had a family history for glucose metabolism alterations and was born to woman with impaired glucose tolerance, gestational diabetes mellitus, hypertension, or obesity. No child presented with congenital anomalies, psychomotor delay, and chronic/autoimmune disease, was taking any medication or was involved in programmed physical activity and/or dietary programs.
According to BW, the study cohort was divided into three categories: AGA children, defined as neonates whose BW was between the 10th and 90th percentile for gestational age; SGA children, defined as neonates whose BW was less than or equal to −2 standard deviation (SD), with evidence of catch-up growth; and LGA children, defined as neonates whose BW was greater than the 90th percentile for gestational age.
A physical examination, including anthropometric measurements (height, weight), was performed and basal blood pressure was measured in all children. The pubertal stage was defined according to the Tanner criteria (all subjects were prepubertal, corresponding to stage 1). Fasting blood samples were obtained to measure fasting glucose, IR-indexes (FI and HOMA-IR), and lipid profile (total cholesterol, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, and triglycerides [TG]). In addition, multiple aliquots of fasting blood samples were collected and stored at −80°C for later assessment of ADMA and NO.
The study was approved by the Ethics Committee of the University of Chieti. Written informed parental consent and oral assent from children were obtained.
Anthropometric measurements
Body weight was determined to the nearest 0.1 kg, and height was measured with a Harpenden stadiometer to the nearest 0.1 cm. BMI was calculated as weight in kilograms divided by the square of the height in meters.
Blood pressure
Systolic blood pressure as well as diastolic blood pressure were measured three times from the nondominant arm after 5 min of rest in a supine position by using a calibrated sphygmomanometer and averaged. The cuff size, which was based on the length and circumference of the upper arm, was chosen to be as large as possible without having the elbow skin crease obstructing the stethoscope. We used an inflatable bladder width that was at least 40% of the arm circumference at a point midway between the olecranon and acromion and was long enough to cover 80–100% of the circumference of the arm.
Biochemical analysis
The plasma glucose level was determined by using the glucose oxidase method, and plasma insulin was measured with a two-site immunoenzymometric assay (AIAPACK IRI; Tosoh, Tokyo, Japan). The limit of detection was 0.5 μU/mL with intra- and inter-assay coefficients of variation of <7% for quality control. HOMA-IR was calculated with the following formula: FI (μU/mL)×fasting glucose (mM)/22.5.
Lipid analysis
Serum total cholesterol, HDL cholesterol, and TG concentrations were determined by the calorimetric enzymatic method. LDL cholesterol was calculated according to the Friedewald formula (LDL cholesterol=total cholesterol−HDL cholesterol−TG/5).
ADMA determination
The plasma ADMA concentration was determined by an ELISA kit (DLD Diagnostika, Hamburg, Germany). In brief, cross reactivity was 1.2% for symmetric dimethylarginine and <0.02% for l-arginine. The limit of detection was 0.05 μM. The intra- and the inter-assay variation was 5.7 and 8.6 coefficients of variation %, respectively. There is a good correlation of the values measured by this enzyme linked immunosorbent assay and liquid chromatography-tandem mass spectrometry (n=29; r=0.984; p<0.0001).
NO determination
Serum NO was measured in triplicate using a classical two-step assay. In detail, NO was defined by the sum of its metabolites, nitrite and nitrate, defined following the conversion of nitrate to nitrite using a commercially available kit based on the Griess reaction (Total Nitric Oxide Assay kit; Assay Design, Ann Arbor, MI). The consumption of foods containing nitrates (i.e., spinach, beet, cabbage, cauliflower, beetroot, and lettuce, among others) was discouraged for the 48 h preceding the measurements and evaluated by a 2-day dietary diary to avoid any dietary influence on the results of the NO assay.
Statistical analysis
According to BW, all children were divided into three groups (AGA, SGA, and LGA). All values are expressed as mean±SD unless otherwise stated. Variables of interest non-normally distributed were logarithmically transformed (FI, HOMA-IR, TG). Differences in variables among the three groups were analyzed by one-way analysis of variance with the Bonferroni's test for post hoc comparison of means between each pair of groups. Differences in the sex variable were analyzed by the Fisher's exact test. A multiple linear regression analysis was performed to evaluate the independent contribution of BW as a categorical variable (AGA, SGA, and LGA) and IR on ADMA levels. In a first model, logADMA was the dependent variable and BW categories and logFI were the independent variables, whereas in a second model, logADMA was the dependent variable and BW categories and logHOMA-IR were the independent variables. In addition, a multiple linear regression analysis was performed to assess the independent contributions of BW categories on NO. All these associations were adjusted for age, sex, and BMI SDS. To include the BW categories as predictors, dummy coding was used. p Values <0.05 were considered statistically significant. SPSS program version 16.0 for Windows was used.
Power calculation
To estimate the power of this study, with a sample size of 27 subjects for each group, assuming α=0.05, and with the aim of detecting a 50% difference between each pair of groups, the power was of 95% to detect a 50% difference in ADMA and of 90% for detecting the same difference in NO. Our estimate of a 50% difference between each pair of groups was based on data from the literature (8).
Abbreviations Used
- ADMA
asymmetric dimethylarginine
- AGA
appropriate for gestational age
- BMI
body mass index
- BW
birth weight
- DBP
diastolic blood pressure
- DDAH
dimethylarginine dimethylaminohydrolase
- FI
fasting insulin
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model assessment of insulin resistance
- IR
insulin resistance
- LDL
low-density lipoprotein
- LGA
large for gestational age
- NO
nitric oxide
- NOS
nitric oxide synthase
- PRMTs
protein arginine methyltransferases
- SBP
systolic blood pressure
- SD
standard deviation
- SDS
standard deviation score
- SGA
small for gestational age
- TG
triglycerides
References
- 1.Böger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr 134: 2842S–2847S, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bouras G, Deftereos S, Tousoulis D, Giannopoulos G, Chatzis G, Tsounis D, Cleman MW, and Stefanadis C. Asymmetric dimethylarginine (ADMA): a promising biomarker for cardiovascular disease? Curr Top Med Chem 13: 180–200, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Chiavaroli V, Giannini C, D'Adamo E, de Giorgis T, Chiarelli F, and Mohn A. Insulin resistance and oxidative stress in children born small and large for gestational age. Pediatrics 124: 695–702, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Franco MC, Higa EM, D'Almeida V, de Sousa FG, Sawaya AL, Fortes ZB, and Sesso R. Homocysteine and nitric oxide are related to blood pressure and vascular function in small-for-gestational-age children. Hypertension 50: 396–402, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Norris SA, Osmond C, Gigante D, Kuzawa CW, Ramakrishnan L, Lee NR, Ramirez-Zea M, Richter LM, Stein AD, Tandon N, Fall CH, and COHORTS Group. Size at birth, weight gain in infancy and childhood, and adult diabetes risk in five low- or middle-income country birth cohorts. Diabetes Care 35: 72–79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisaneschi S, Strigni FAL, Sanchez AM, Begliuomini S, Casariosa E, Ripoli A, Ghirri P, Boldrini A, Fink B, Genazzani AR, Coceani F, and Simoncini T. Compensatory feto-placental upregulation of the nitric oxide system during fetal growth restriction. PLoS One 7: e45294, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renom Espineira A, Fernandes-Rosa FL, Bueno AC, de Souza RM, Moreira AC, de Castro M, Barbieri MA, Bettiol H, and Antonini SR. Postnatal growth and cardiometabolic profile in young adults born large for gestational age. Clin Endocrinol (Oxf) 75: 335–341, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Tain YL. and Huang LT. Asymmetric dimethylarginine: clinical applications in pediatric medicine. J Formos Med Assoc 110: 70–77, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Vida G, Sulyok E, Lakatos O, Ertl T, Martens-Lobenhoffer J, and Bode-Böger SM. Plasma levels of asymmetric dimethylarginine in premature neonates: its possible involvement in developmental programming of chronic diseases. Acta Paediatr 98: 437–441, 2009 [DOI] [PubMed] [Google Scholar]