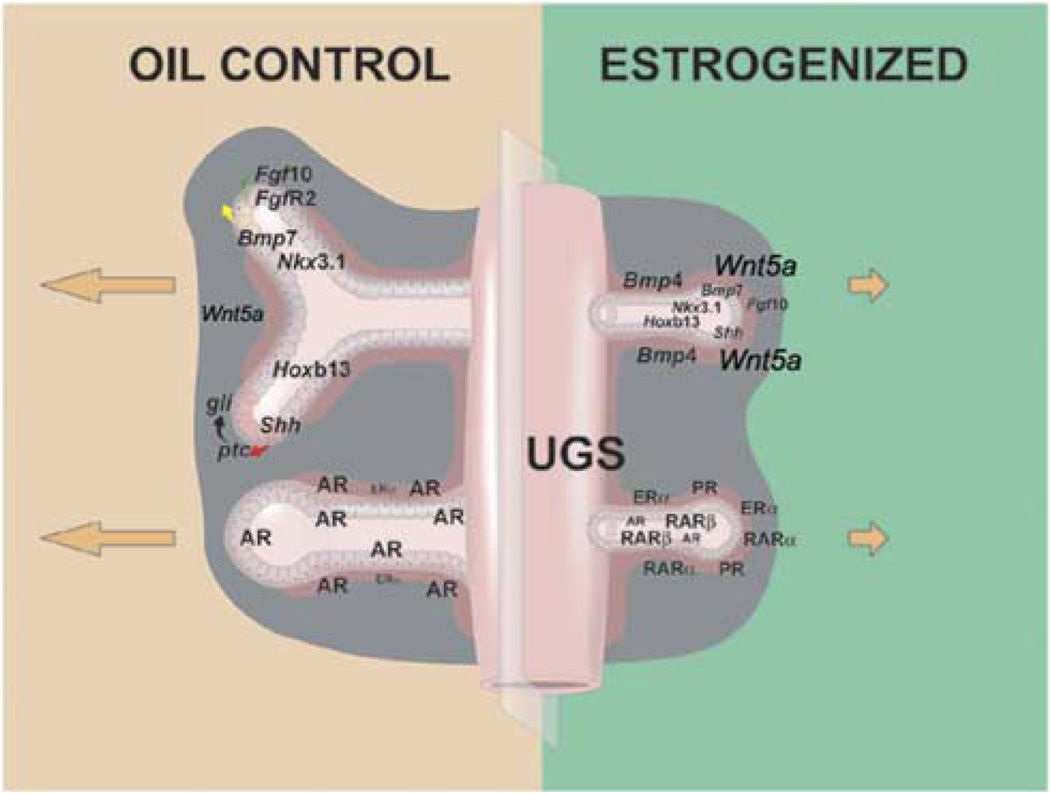
Fig. 1.
A schematic representation of the neonatal estradiol-induced organizational and gene expression changes found in the developing rat prostate lobes. Relative gene expression levels between control and treated prostates are represented by type size. In the normal prostate (left panel), androgen receptor (AR) is the dominant steroid receptor dictating morphogenesis in the branching and differentiating structure. Lower amounts of ERα are found in the proximal ducts. Together, these steroids influence the expression of multiple morphoregulatory genes that drive ductal outgrowth and branching. Mesenchymal expression of Fgf10 acts on epithelial FgfR2 receptors to stimulate branching while mesenchymal Wnt5a and Bmp4 act as localized inhibitors to restrict growth at specific sites. Epithelial cells secrete Shh, which acts on mesenchymal ptcreceptors that in turn activate the gli transcription factors to direct growth at the distal tips of emerging ducts. Transcription factors Nkx3.1 and Hoxb13 are expressed by developing epithelial cells and control early differentiation pathways. Neonatal exposure to high-dose estradiol suppresses ductal outgrowth and branching morphogenesis and perturbs differentiation through alterations in these signaling networks (right panel). The structural alterations are initiated through changes in steroid receptor expression in the developing gland including reduced AR, amplified ERα, induction of progesterone receptor (PR) and increased RARα, β and γ expression (lower right schematic). The alterations in steroidal signaling immediately redirect the expression of morphoregulatory genes (upper right schematic). Increased Bmp4 and Wnt5a combined with reduced Fgf10 expression suppress growth and branching of the ducts. Transient reduction in Nkx3.1 and permanent downregulation of Hoxb13 initiate epithelial differentiation defects that persist throughout life and predispose to neoplasia with aging.