Abstract
Noroviruses (NoVs) are important cause of gastroenteritis in humans worldwide. Genotype GII.4 is responsible for the majority of outbreaks reported to date. This study describes, for the first time in Brazil, the circulation of NoV GII.4 variant Sydney 2012 in faecal samples collected from children aged less than or equal to eight years in Rio Branco, state of Acre, northern Brazil, during July-September 2012.
Keywords: norovirus, gastroenteritis, GII.4 variant
Noroviruses (NoVs) are the major cause of epidemic viral gastroenteritis and the leading cause of foodborne outbreaks in diverse countries in the world. The disease in most cases occurs with diarrhoea and vomiting, affecting mainly children, the elderly and immunocompromised persons. These viruses are often associated with outbreaks in closed settings such as schools, hospitals, hotels, cruise ships and nursing homes (Green 2007).
The importance of NoV as agents of childhood gastroenteritis extends throughout the developing countries since the late 1970s, being considered the second causative agent of viral gastroenteritis in children under five years old (Rackoff et al. 2013). NoVs possess an icosahedral virion of 27-32 nm in diameter, single stranded, positive-sense, polyadenylated RNA genome of 7,400-7,700 nucleotides (Kapikian et al. 1972). The viral genome is organised into three open reading frames (ORFs). ORF1 encodes the non-structural proteins, including the RNA-dependent RNA polymerase (Clarke & Lambden 2000). The ORF2 encodes the structural capsid protein VP1, the main component of the viral capsid, divided into three major structural domains: S, P1 and P2. The P2 domain contains the most variable sequence and is located on the surface of the capsid, playing an important role in immune recognition and receptor interaction (Prasad et al. 1999, Glass et al. 2000).
NoVs exhibiting a large genetic diversity and are classified into five genogroups (GI-GV) based on VP1 amino acid sequence and these are further subdivided into multiple genotypes (Zheng et al. 2006). Human infection is associated with GI, GII and GIV genogroups, of which the GII.4 genotype has maintained importance over more than 30 years in both outbreaks and sporadic cases (Siebenga et al. 2009, Rackoff et al. 2013). Diverse GII.4 variants have been associated with global epidemics of acute gastroenteritis from 1996 to the present, including the US 1995/96 variant in 1996 (White et al. 2002), Farmington Hills variant in 2002 (Widdowson et al. 2004), Hunter variant in 2004 (Bull et al. 2006), 2006a and 2006b variant in 2007-2008 (Eden et al. 2010), New Orleans variant in 2009-2012 (Yen et al. 2011) and most recently Sydney 2012 (van Beek et al. 2013).
The first report of Sydney 2012 variant was in March 2012 in New South Wales, Australia (van Beek et al. 2013). Thereafter, it was detected in the United States of America (USA) in September 2012 (Barclay et al. 2013), in Belgium, September and December 2012, and in Denmark in November 2012 (Fonager et al. 2013). In this period, various countries, such as New Zealand, France, Scotland, Japan, Hong Kong and the USA, have reported a higher incidence of NoV outbreaks (Barclay et al. 2013, Bennett et al. 2013, Chan & Chan 2013). According to published data obtained by NoroNet, there are suggestive evidences that this increase is related to this new NoV GII.4 2012 variant (van Beek et al. 2013).
This study analysed 25 stool samples of sporadic cases of acute gastroenteritis among hospitalised children which were obtained within the National Surveillance Program of Rotavirus Gastroenteritis coordinated by the Brazilian Ministry of Health. This program comprises three officials Reference Centres and Evandro Chagas Institute is one of them.
The detection of NoV was first performed using a commercial enzyme immunoassay (EIA) (Ridascreen(r) Norovirus 3rd Generation, R-Biopharm AG, Darmstadt, Germany) following the manufacturer's instructions. Viral RNA was extracted from a 10% faecal suspension (the same used in the EIA test) using a guanidine isothiocyanate/silica method (Boom et al. 1990) followed by cDNA synthesis performed using a pd(N)6TM random primer (Amersham Biosciences, UK) and the SuperscriptTM II RNAse H Reverse Transcriptase (Invitrogen, USA).
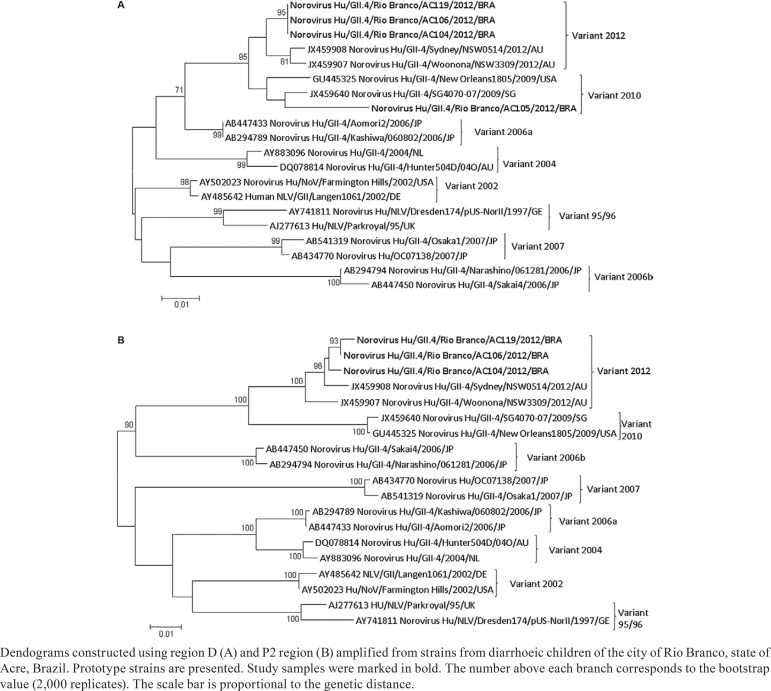
Initially, the viral genome was amplified by reverse transcription polymerase chain reaction (RT-PCR) using primers for B region of the polymerase gene (ORF1) (Anderson et al. 2001, Fankhauser et al. 2002). For genetic characterisation were used primers for region D (ORF2) of the viral capsid of NoV GII with primers Cap C, Cap D1 and Cap D3 (Vinjé et al. 2004). In addition, these samples were tested with primers for P2 region (EVP2F and EVP2R) described to define GII.4 variants (Vega et al. 2011). The amplicons obtained were purified with a QIAquick(r) PCR Purification Kit and QIAquick(r) gel Purification Kit (QIAGEN, Valencia, CA, USA) following the manufacturer's recommendations. DNA sequencing was performed using the ABI Prism 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The sequences were edited using the BioEdit Sequence Alignment Editor (v.7.0.9.1) software. The evolutionary history was inferred using the neighbour-joining method, bootstrap 2,000 replicates and model using MEGA 5.1 (Saitou & Nei 1987, Tamura et al. 2011).
From July-September 2012, 25 samples were collected by the Surveillance Program from children with acute gastroenteritis the city of Rio Branco, state of Acre. Patient's ages ranged from four month-eight years (40%, 0-12 months; 24%, 1-2 years old; 28% > 2 years old; 8% no information). These samples were analysed by EIA and RT-PCR using region B with a positivity of 48% (12/25) in both, but one sample was positive only by EIA and another only by RT-PCR. All positive cases were shown to have diarrhoea as the major symptom, with duration of four days on average; there were no other symptom recorded on medical histories sent to us.
NoVs genotypes were characterised in four samples using primers for the capsid region (region D). The New Orleans 2009 was detected in one sample and Sydney 2012 GII.4 variants in three samples. The analyses of P2 region confirmed the presence of the Sydney 2012 GII.4 variant and the sequences obtained had a high nucleotide identity among them (range 98.8-99.5%) and differed in the range of 1.2-2.6% when compared with the prototype strain (Figure). These sequences were submitted to GenBank under accessions KF360222-KF360228.
Briefly, this study demonstrated by phylogenetic analysis the circulation of the two most recently identified GII.4 variants, New Orleans 2009 and Sydney 2012 in Rio Branco and, to our knowledge, this represents the first detection of the recently emerged GII.4 Sydney 2012 variant in Brazil.
Several studies demonstrate that Sydney 2012 variant has the potential for strain replacement and can rapidly diversify within the population, a phenomenon driven by evolutionary forces and leading to persistence of the GII.4 variants in a community (Eden et al. 2010, Fonager et al. 2013). As a consequence, a rapid worldwide dispersion of the GII.4 variants may occur; nevertheless, further studies are needed in order to better assess this issue, including its association with gastroenteritis outbreaks.
Our results warrant additional studies in other settings in Brazil to see whether this variant is spreading across the country.
In addition, further local studies are needed, including a larger sample size to better evaluate epidemiological and molecular aspects related to the emergence of this new GII.4 2012 variant.
ACKNOWLEDGEMENTS
To the support provided by Dielle Monteiro Teixeira, Euzeni Menezes and all students and technical of the Norovirus and Astrovirus Laboratory, and to the Health Department of Acre State, for the samples.
Footnotes
Financial support: IEC
REFERENCES
- Anderson AD, Garrett VD, Sobel J, Monroe AS, Fankhauser RL, Schwab KJ, Bresee JS, Mead PS, Higgins C, Campana J, Glass RI. Multistate outbreak of Norwalk-like virus gastroenteritis associated with a common cateter. Am J Epidemiol . 2001;11:1013–1019. doi: 10.1093/aje/154.11.1013. [DOI] [PubMed] [Google Scholar]
- Barclay L, Wikswo M, Gregoricu N, Vinjé J, Lopman B, Parashar U, Hal A. Emergence of new norovirus strain GII.4 Sydney-United States, 2012. MMWR Morb Mortal Wkly Rep . 2013;62:55–55. [PMC free article] [PubMed] [Google Scholar]
- Bennett S, MacLean A, Miller RS, Aitken C, Gunson RN. Increased norovirus activity in Scotland in 2012 is associated with the emergence of a new norovirus GII.4 variant. Euro Surveill . 2013;18: [PubMed] [Google Scholar]
- Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PME, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol . 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull RA, Tu ET, McIver CJ, Rawlinson WD, White PA. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J Clin Microbiol . 2006;44:327–333. doi: 10.1128/JCM.44.2.327-333.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MC, Chan PK. Complete genome sequence of a novel recombinant human norovirus genogroup II genotype 4 strain associated with an epidemic during summer of 2012 in Hong Kong. Genome Announc . 2013;1:e00140–e00112. doi: 10.1128/genomeA.00140-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IN, Lambden PR. Organization and expression of calicivirus genes. J Infect Dis . 2000;181(Suppl.):S309–S316. doi: 10.1086/315575. [DOI] [PubMed] [Google Scholar]
- Eden JS, Bull RA, Tu E, McIver CJ, Lyon MJ, Marshall JA, Smith DW, Musto J, Rawlinson WD, White PA. Norovirus GII.4 variant 2006b caused epidemics of acute gastroenteritis in Australia during 2007 and 2008. J Clin Virol . 2010;49:265–271. doi: 10.1016/j.jcv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Fankhauser RL, Monroe SS, Noel JS, Humphrey CD, Bresee JS, Parashar UD, Ando T, Glass RI. Epidemiological and molecular trends of "Norwalk-like viruses" associated with outbreaks of gastroenteritis in the United States. J Infect Dis . 2002;186:1–7. doi: 10.1086/341085. [DOI] [PubMed] [Google Scholar]
- Fonager J, Hindbak LS, Fischer TK. Rapid emergence and antigenic diversification of the norovirus 2012 Sydney variant in Denmark, October to December, 2012. Euro Surveill . 2013;18:20413–20413. [PubMed] [Google Scholar]
- Glass PJ, White LJ, Ball JM, Leparc-Goffart I, Hardy ME, Estes MK. Norwalk virus open reading frame 3 encodes a minor structural protein. J Virol . 2000;74:6581–6591. doi: 10.1128/jvi.74.14.6581-6591.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KY. Fields BN. Fields virology . 5th ed. Lippincott, Williams & Wilkins; Philadelphia: 2007. Caliciviridae: the noroviruses; pp. 949–979. [Google Scholar]
- Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, Chanock RM. Visualization by immune electron microscopy of a 27 nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol . 1972;10:1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. X-ray crystallographic structure of the Norwalk virus capsid. Science . 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- Rackoff LA, Bok K, Green KY, Kapikian AZ. Epidemiology and evolution of rotaviruses and noroviruses from an archival WHO Global Study in Children (1976-79) with implications for vaccine design. PLoS ONE . 2013;8: doi: 10.1371/journal.pone.0059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The Neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol . 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Siebenga JJ, Vennema H, Zheng DP, Vinje J, Lee BE, Pang XL, Ho ECM, Lim W, Choudekar A, Broor S, Halperin T, Rasool NBG, Hewitt J, Greening GE, Jin M, Duan ZJ, Lucero Y, O'Ryan M, Hoehne M, Schreier E, Ratcliff RM, White PA, Iritani N, Reuter G, Koopmans M. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001-2007. J Infect Dis . 2009;200:802–812. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol . 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek J, Ambert-Balay K, Botteldoorn N, Eden JS, Fonager J, Hewitt J, Iritani N, Kroneman A, Vennema H, Vinjé J, White PA, Koopmans M. NoroNet indications for worldwide increased norovirus activity associated with emergence of a new variant of genotype II.4, late 2012. Euro Surveill . 2013;18:8–9. [PubMed] [Google Scholar]
- Vega E, Barclay L, Gregoricus N, Williams K, Lee D, Vinjé J. Novel surveillance network for norovirus gastroenteritis outbreaks, United States. Emerg Infect Dis . 2011;17:1389–1395. doi: 10.3201/eid1708.101837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinjé J, Hamidjaja RA, Sobsey MD. Development and application of a capsid VP1 (region D) based reverse transcription PCR assay for genotyping of genogroup I and II noroviruses. J Virol Methods . 2004;116:109–117. doi: 10.1016/j.jviromet.2003.11.001. [DOI] [PubMed] [Google Scholar]
- White PA, Hansman GS, Li A, Dable J, Isaacs M, Ferson M, McIver CJ, Rawlinson WD. Norwalk-like virus 95/96-US strain is a major cause of gastroenteritis outbreaks in Australia. J Med Virol . 2002;68:113–118. doi: 10.1002/jmv.10177. [DOI] [PubMed] [Google Scholar]
- Widdowson MA, Cramer EH, Hadley L, Bresee JS, Beard RS, Bulens SN, Charles M, Chege W, Isakbaeva E, Wright JG, Mintz E, Forney D, Massey J, Glass RI, Monroe SS. Outbreaks of acute gastroenteritis on cruise ships and on land: identification of a predominant circulating strain of norovirus - United States, 2002. J Infect Dis . 2004;190:27–36. doi: 10.1086/420888. [DOI] [PubMed] [Google Scholar]
- Yen C, Wikswo ME, Lopman BA, Vinje J, Parashar UD, Hall AJ. Impact of an emergent norovirus variant in 2009 on norovirus outbreak activity in the United States. Clin Infect Dis . 2011;53:568–571. doi: 10.1093/cid/cir478. [DOI] [PubMed] [Google Scholar]
- Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology . 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]