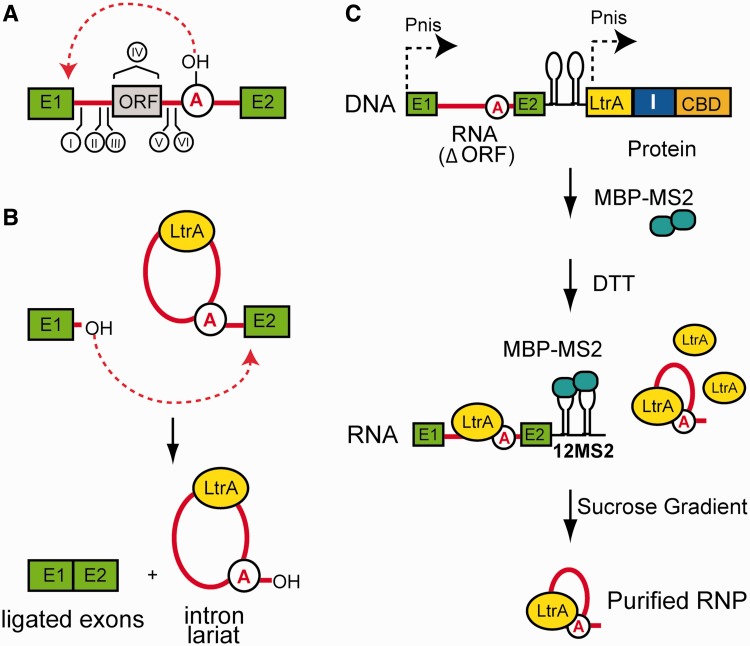
Figure 1.
Domain structure, activity and purification of native group II introns. (A) Domain structure of a group II intron. Rendered as squares are the exons (E1 and E2) flanking the group II intron sequence. Denoted with Roman numerals are the six structural domains of the intron. Domain IV contains an ORF that encodes an IEP, also referred to as LtrA. Domain VI contains the catalytic adenosine nucleophile (circled) required for splicing. (B) Splicing reaction carried out by group II introns. Group II introns are excised from flanking exons through a two-step process that is catalyzed by the intron RNA. (C) Purification strategy. Lysates from nisin-induced cells are passed over a chitin resin column to capture LtrA–intein–CBD protein fusion and associated RNA, via a C-terminal chitin-binding domain in the LtrA fusion. I = intein. To facilitate separation (see below), MBP-MS2 (turquoise balls) is added to bind the MS2 tag in the precursor at the 3′ exon (black lollipops). Cleavage of LtrA from the intein with DTT) releases the RNP from the chitin column in both active and precursor forms. Assuming a 1:1 stoichiometry between MBP-MS2 protein (∼50 kD) and its RNA-binding site (21), addition of the MS2 binding protein further increases the size differential between the 12xMS2-containing precursor (∼1.4 MDa) and the spliced lariats form (∼430 kD) of the RNP. This large size differential allows for successful final isolation of the active RNP from the precursor species by sedimentation, using a sucrose cushion gradient.