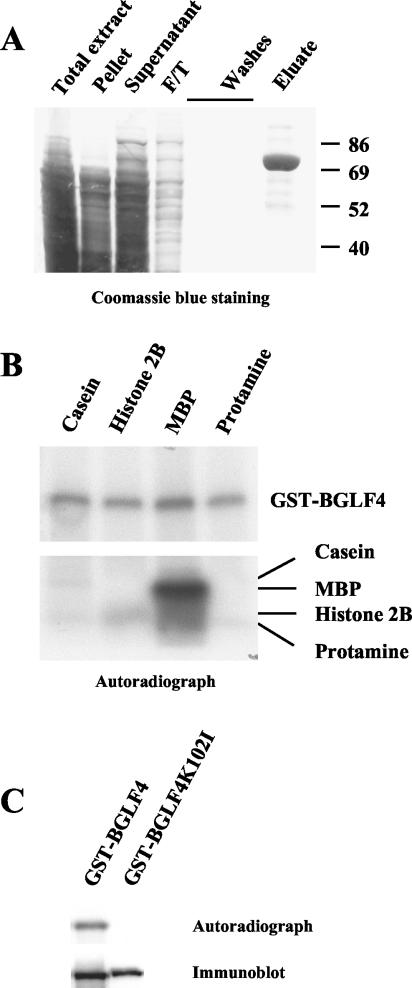
FIG. 2.
Purification and kinase activity of EBV PK. (A) EBV PK was expressed in insect cells infected with recombinant baculovirus and purified following a published protocol (12). The purified protein appears as a single band at ∼78 kDa detected by Coomassie staining. Numbers on the right side of the gel are standard molecular weights in thousands. (B) Kinase activity of the purified EBV PK was examined in in vitro kinase assays with casein, MBP, histone 2B, and protamine as substrates (lower panel) or in autophosphorylation assays (upper panel). The whole reaction mixtures were separated by SDS-PAGE (4 to 20% gradient gel), and dried gels were exposed to X-ray film. No labeled bands were detected in mock reactions (without kinase) (data not shown). (C) Specificity of the autophosphorylation was confirmed by use of the kinase-null K102I mutant of EBV BGLF4. The kinase assays were performed as described before, and the reaction mixtures were extensively washed, subjected to SDS-PAGE, and exposed to X-ray film (upper panel), followed by immunoblotting with anti-GST antibody (bottom panel).