Figure 1.
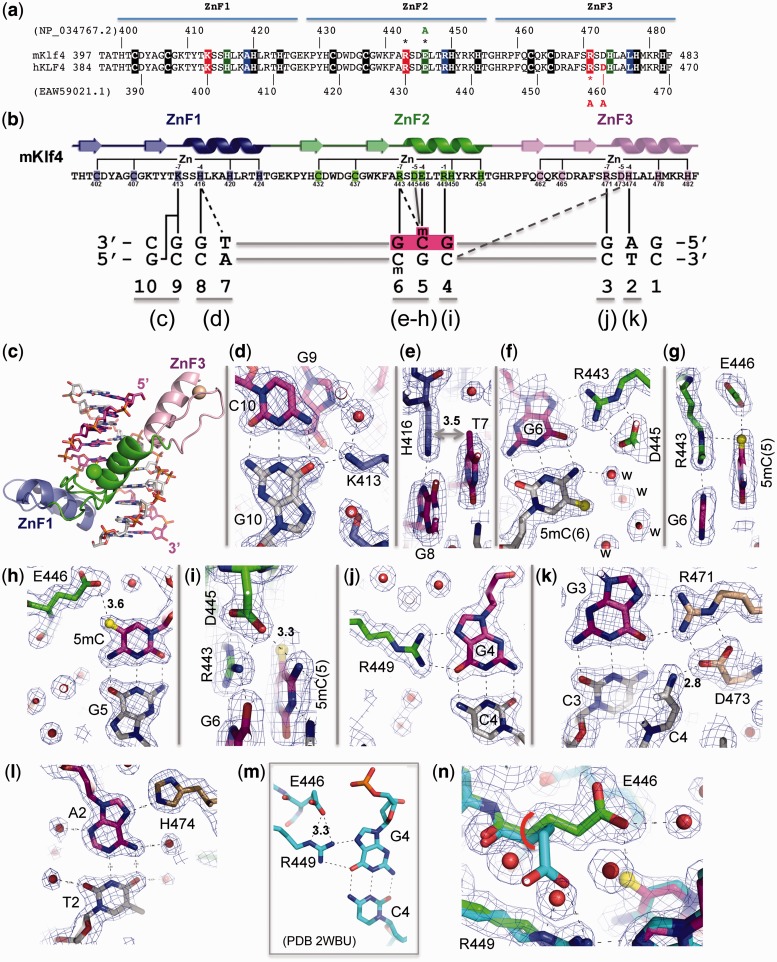
Klf4 binds methylated CpG. (a) Sequence alignment of the C-terminal ZnF DNA-binding domains of mouse Klf4 (mKlf4) and human KLF4 (hKLF4), which are identical in sequence. The mutations made by Hu et al. (22), R458A and D460A of hKLF4 are located in the last (third) ZnF, which does not directly participate in methyl-CpG binding. (b) Schematic representation of mKlf4 DNA-binding ZnF domain. The sequence and the secondary structure are shown as follows: (arrows) β strands and (ribbons) α helices. The positions highlighted are responsible for Zn ligand binding (C2H2) and DNA base-specific interactions at −1, −4, −5 and −7 positions (relative to the first zinc-binding histidine): solid lines (direct hydrogen bonds) and dashed lines (van der Waals contacts). The DNA sequence used for the study is shown with the majority of base interactions involving the top strand from 3′-to-5′ (left-to-right). The central GCG sequence is colored in magenta and the letter ‘m’ indicates the methyl group in 5mC. Dotted and solid vertical lines indicate specific binding interactions. (c) The mKlf4 ZnF protein binds in the major groove of DNA with ZnF1 (blue), ZnF2 (green) and ZnF3 (pink). (d) Lys413 of ZnF1 at the −7 position interacts with the O6 oxygen atoms of both guanines at G9 (of upper strand) and G10 (of lower strand). (e) His416 of ZnF1 at the −4 position interacts with the TpG dinucleotide. (f) Arg443–Gua6 interaction; a layer of ordered water molecules (marked ‘w’) shields the methyl group of lower strand 5mC. (g) The upper strand 5mCpG interacts with Arg443 and forms a 5mC-Arg-Gua triad. (h) One of the carboxylate oxygen atoms of Glu446 forms a weak C-H…O type of hydrogen bond with the methyl group of the upper strand 5mC. (i) Asp445 of ZnF2 at the −5 position interacts with Arg443 at the −7 position and the N4 atom of 5mC of the upper strand. (j) Arg449–G4 interaction. (k) Arg471–G3 interaction; Asp473 of ZnF3 at the −5 position interacts with Arg471 at the −7 position and the N4 atom of Cyt4 of the lower strand. (l) His474–A2 interaction. (m and n) Structural comparison of mKlf4 Glu446 in the absence (m) and presence of methylation (n).