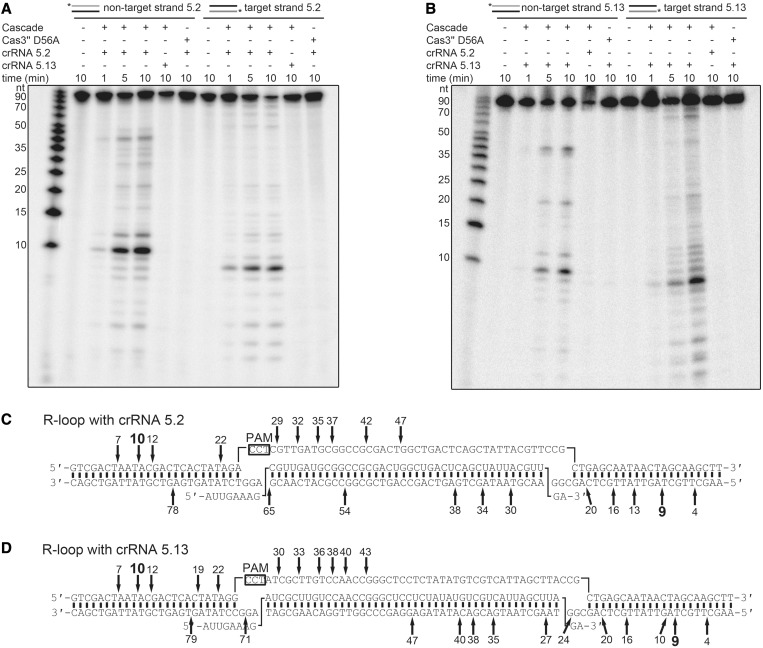
Figure 4.
Interference activity of in vitro assembled type I-A Cascade. (A) The assembled Cascade complex is loaded with crRNA 5.2 for 20 min at 70°C, and the interference reaction is started with the addition of ATP, Mg2+, Mn2+ and the dsDNA substrate (in_5.2 CCT), which is either 5′-[γ-32P]-ATP labeled on the non-target (forward) or the crRNA target strand (reverse). Cleavage reactions were stopped at three different time points (1, 5, 10 min at 70°C). The reaction products of the cleaved dsDNA were separated on 20% denaturing gels. The non-matching crRNA 5.13 and the Cas3′′ D56A mutant are used as controls. (B) In parallel, the crRNA 5.13 is loaded into Cascade, and cleavage of the matching dsDNA substrate (in_5.13 CCT) is visualized. (C and D) The cleavage products are analyzed on 10% Urea-PAGE for each strand (in_5.2 CCT for/rev and in_5.13 CCT for/rev) with two different markers (Supplementary Figure S9). The cleavage sites are marked within the proposed R-loop structure that is formed during the interference reaction [(C) dsDNA 5.2, (D) dsDNA 5.13].