Figure 1.
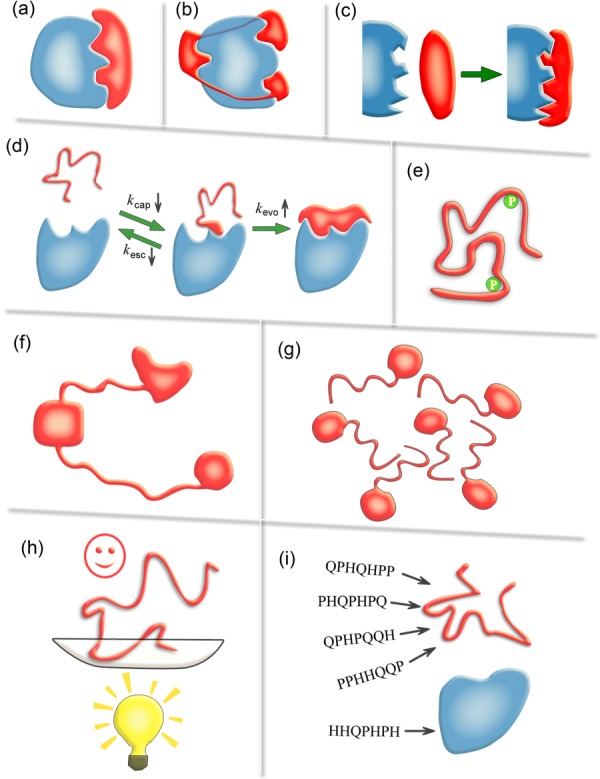
Schematic diagrams illustrating nine possible advantages of IDPs/IDRs. (a) Economizing genome and protein resources: IDPs/IDRs use smaller protein size to afford the same interface area as ordered proteins. (b) Overcoming steric restrictions in binding: IDPs/IDRs can overcome steric restrictions in binding complexes by protruding into partners or wrapping around them in ways difficult for ordered proteins. (c) Achieving high specificity with low affinity: the highly complementary binding interfaces and the unfavorable conformational-entropy changes result in high specificity and low affinity. (d) Increasing binding rate: the kinetic advantage of IDPs in a binding process stems from a faster evolution step from the encounter complex and a slower escaping rate. (e) Facilitating posttranslational modifications: the conformational flexibility of IDPs/IDRs greatly facilitates the exposure of modification sites and their binding to modifying enzymes. (f) Enabling flexible linkers: the lack of ordered structures makes IDPs/IDRs dominant in flexible linkers of proteins which are obviously out of reach of ordered proteins. (g) Preventing aggregation: IDPs/IDRs possess favorable interactions with water and are inherently advantageous in preventing aggregation. (h) Providing resistance to non-native conditions: you cannot unfold what is already unfolded. (i) Allowing compatibility with more available sequences: the sequence space of IDPs/IDRs is expected to be larger than that of ordered proteins.
