Figure 1.
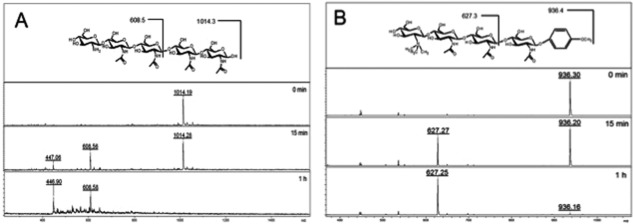
Enzyme activity of ChitA on modified chitin substrates. A: Enzyme activity of ChitA on the modified chitopentaose substrate GlcN-GlcNAc4, which contains a non-acetylated glucosamine residue at the nonreducing end (structure shown at top). The disappearance of the substrate (m/z 1014.4) and appearance of cleavage products (GlcN-GlcNAc2, m/z 608.5; and chitobiose, m/z 447.0) as monitored by MALDI-TOF MS are apparent over the assay time course, with the [M + Na]+ masses revealing the cleavage position. B: Enzyme activity of ChitA on the modified chitotetraose substrate TMG-chitotriomycin-pMP, which contains a N-trimethylamino glucosamine (TMG) residue at the non-reducing end, and a p-methyoxyphenyl protecting group at the anomeric position (structure shown at top). The disappearance of the substrate (m/z 937.4) and appearance of a cleavage product (TMG-GlcNAc2, m/z 627.3) as monitored by MALDI-TOF mass spectrometry is apparent over the assay time course, with the [M + Na]+ masses revealing the cleavage position.
