Abstract
AC98-6446 is a novel semisynthetic derivative of a natural product related to the mannopeptimycins produced by Streptomyces hygroscopicus. Naturally occurring esterified mannopeptimycins exhibited excellent in vitro activity but only moderate in vivo efficacy against staphylococcal infection. The in vivo efficacy and pharmacokinetics of AC98-6446 were investigated in murine acute lethal, bacterial thigh and rat endocarditis infections. Pharmacokinetics were performed in mice, rats, monkeys, and dogs. Acute lethal infections were performed with several gram-positive isolates: Staphylococcus aureus (methicillin-susceptible and methicillin-resistant staphylococci), vancomycin-resistant Enterococcus faecalis, and penicillin-susceptible and -resistant Streptococcus pneumoniae. The 50% effective dose for all isolates tested ranged from 0.05 to 0.39 mg/kg of body weight after intravenous (i.v.) administration. Vancomycin was more than fivefold less efficacious against all of these same infections. Results of the thigh infection with S. aureus showed a static dose for AC98-6446 of 0.4 mg/kg by i.v. administration. Reduction of counts in the thigh of >2 log10 CFU were achieved with doses of 1 mg/kg. i.v. administration of 3 mg/kg twice a day for 3 days resulted in a >3 log10 reduction in bacterial counts of vancomycin-susceptible and -resistant E. faecalis in a rat endocarditis model. Pharmacokinetics of AC98-6446 showed an increase in exposure (area under the concentration-time curve) from mouse to dog species. The i.v. half-life (t1/2) increased threefold between rodents and the higher species dosed. Efficacy of AC98-6446 has been demonstrated in several models of infection with resistant gram-positive pathogens. This glycopeptide exhibited bactericidal activity in these models, resulting in efficacy at low doses with reduction in bacterial load.
Staphylococci and enterococci are the cause of many hospital and community-acquired infections. The severity of these infections can range from skin and skin and skin structure infections to septic arthritis, osteomyelitis, and prosthetic valve endocarditis (9, 12).
The methicillin-resistant staphylococci (MRSA) are prevalent in most major medical centers, compromising a large percentage of staphylococcal infections encountered. The incidence of these isolated pathogens has continued to increase. Their prevalence varies by geographic regions, but they remain a clinical problem due to multidrug resistance (22). Decreased susceptibility to erythromycin, gentamicin, tetracycline, rifampin, and the fluoroquinolones severely limits the therapeutic options for treatment of patients with severe MRSA infections (6). Glycopeptide-intermediate Staphylococcus aureus strains were first identified in the late 1990s, with the first reported vancomycin-resistant strain recently isolated from a patient in a Michigan medical center (14). Staphylococcal strains with reduced susceptibility to vancomycin have now been identified in several countries (3, 13).
Glycopeptide resistance in enterococci is recognized as a clinical problem of increasing importance (7). In particular, vancomycin-resistant Enterococcus (VRE) represents an important nosocomial pathogen capable of spread from colonized infected patients to uncolonized ones (30). Since it was first isolated in Europe in 1986, VRE has reached upwards of 26% in isolates tested from intensive care units in the United States (2). The intrinsic resistance of the enterococci precludes the use of conventional antibiotic therapy (1, 21).
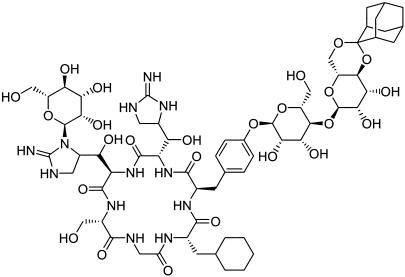
AC98-6446 (Fig. 1), the ketal derivative of a core-modified mannopeptimycin, is a semisynthetic glycopeptide derived from Streptomyces hygroscopicus (8, 28). Esterified mannopeptimycins produced from this organism have demonstrated excellent in vitro activity. MICs of 0.03 to 0.06 μg/ml have been exhibited by AC98-6446 against methicillin-susceptible and -resistant staphylococci. In vitro activity against vancomycin-susceptible and -resistant enterococci has been demonstrated at concentrations of 0.12 to 0.25 μg/ml and ≤0.008 μg/ml for Streptococcus pneumoniae (P. Petersen, P. Labthavikul, T. Wang, R. Dushin, and P. Bradford, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-353, 2002). Additionally, AC98-6446 has been shown to be bactericidal, exhibits a long (2 to 4 h) gram-positive postantibiotic effect (P. Petersen, H. Hartman. T. Wang, R. Dushin, and P. Bradford, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-354, 2002), and exhibits limited inoculum effect and activity against adherent cells in a biofilm (P. Labthavikul, P. Petersen, T. Wang, R. Dushin, and P. Bradford, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-355, 2002). These properties make it a potential candidate for treatment of serious infection caused by resistant staphylococci and enterococci.
FIG. 1.
Chemical structure of AC98-6446.
The present study was undertaken to evaluate the in vivo efficacy of AC98-6446 in several experimental infection models with these organisms as well as to assess the pharmacokinetics of the compound in several species.
(Data from this study were presented in part at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 27 to 30 September, 2002 [T. Murphy, E. Lenoy, M. Young, and W. Weiss, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-356, 2002].)
MATERIALS AND METHODS
Organisms.
All organisms used in this study were taken from the Wyeth Research culture collection and represent recent clinical isolates collected from various medical centers around the United States. Standard laboratory strains of S. pneumoniae ATCC 1894 and ATCC 6301 were obtained from the American Type Culture Collection (Rockville, Md.).
Compounds.
AC98-6446 was prepared by Wyeth Research (Pearl River, N.Y.), and vancomycin was obtained from Sigma Chemical Corp. (St. Louis, Mo.). AC98-6446 was prepared in 5% dextrose water, and vancomycin was prepared in sterile saline for all the models performed.
Acute lethal infection model.
Female mice, strain CD-1 (Charles River Laboratories, Kingston, N.Y.), 20 ± 2 g of body weight each, were challenged by intraperitoneal injection of bacterial cells from 5-h broth cultures suspended in either trypticase soy broth or 5% hog gastric mucin. Five animals were infected at each of five i.v.-administered dose levels, covering a twofold dilution series for AC98-6446 and vancomycin for each organism. The bacterial inoculum level for infection was sufficient to result in death of untreated controls within 24 to 48 h. The 7-day survival ratios from three separate tests were pooled for estimation of the 50% effective dose (ED50) by a computerized program for probit analysis (5). All procedures were carried out by using protocols approved by the Wyeth Research Animal Care and Use Committee.
Murine thigh infection model.
Female CD-1 mice (Charles River Laboratories) weighing 18 to 22 g were rendered neutropenic by intraperitoneal injection of Cytoxan (cyclophosphamide) on days −4 (150 mg/kg of body weight) and −1 (100 mg/kg) before infection. On the day of infection, random colonies of Staphylococcus aureus Smith were selected off a trypticase soy agar plate that was incubated for 24 h at 37°C by using the Prompt inoculation system (BBL Microbiology Systems, Sparks, Md.). This was then diluted in trypticase soy broth to yield a final bacterial concentration of 107 CFU/ml. The thigh infection was induced by intramuscular injection of 0.1 ml of the inoculum into the left thigh of each mouse 1.5 h prior to initiation of treatment. AC98-6446 was administered by intravenous injection of 0.2 ml in twofold dilutions ranging from 0.06 to 16 mg/kg with three mice per dose level. Mice were euthanized 24 h after dose administration and thighs were removed, homogenized, and plated for determination of viable bacteria. The limit of detection of bacterial counts was 102 CFU/thigh. Counts were analyzed by using a sigmoid Emax model (WinNonlin Pro, version 3.0; Pharsight Corporation, Mountain View, Calif.).
Rat endocarditis.
Endocarditis was produced in male Wistar rats (250 g; Charles River Laboratories) by insertion of a sealed polyethylene cannula (PE10) through the right carotid artery into the left ventricle; the cannula was sutured in place as a point of adherence for bacterial infection (5, 6, 15). At 48 h after implantation of the cannula, a 5-h bacterial culture was diluted to 105 to 106 CFU/ml in sterile saline, and 1 ml was injected i.v. (5). Isogenic strains of Enterococcus faecalis were used: GC 6181, a vancomycin-susceptible isolate, and GC 6191, its isogenic vancomycin-resistant (VanA) derivative. Inoculum infection concentration was verified by plate counts. Antibacterial treatment was initiated 24 h after bacterial challenge. Treatments were delivered by intravenous administration every 12 h for 3 days. The dose ranges tested were from 1 to 10 mg/kg/day. Untreated control rats received injections of phosphate-buffered saline. Both treated and control rats were euthanized by CO2 inhalation 24 h after the last treatment. Hearts were aseptically removed, weighed, homogenized, and serially diluted in saline for determination of viable bacteria (the limit of detection was 102 CFU/heart), expressed as log10 CFU per heart.
Pharmacokinetics.
The pharmacokinetics of AC98-6446 were investigated in the mouse, rat, dog, and monkey. Female CD-1 mice (Charles River Laboratories) weighing 18 to 22 g were administered a single dose of AC98-6446 i.v. at 20 mg/kg. Blood samples were taken at selected time points by cardiac puncture, and the blood from three mice was pooled for serum collection at each time point. Male Wistar rats (250 g; Charles River Laboratories) received 20 mg of AC98-6446/kg by i.v. injection. Three rats were used for each dose tested. Blood samples were obtained via a surgically implanted jugular cannula at selected time points. Female beagle dogs (9 to 12 kg) and female cynomolgus monkeys (4 to 6 kg) were administered AC98-6446 by i.v. injection of 20 mg/kg. Three animals were used for each species at each dose. Blood samples were collected from the femoral artery for analysis at selected time points.
Sample analysis.
Analysis of the pharmacokinetic samples was performed by LC-MS (HP1100) via direct injection of the plasma sample (10 μl). A gradient of 10 to 60% (0.02% trifluoracetic acid) acetonitrile over 15 min was used in a Prodigy ODS3 (dimensions, 4.6 by 150 mm, 5-μm column; flow rate, 1 ml/min). Detection was performed by using an MSD detector (single ion monitoring at 717.5; fragmentor, 100; gain, 1) (Agilent Technologies, Palo Alto, Calif.). Limit of detection of AC98-6446 was 50 ng/ml. Pharmacokinetic parameters were determined by using the WinNonlin program (WinNonlin Pro, version 3.0; Pharsight Corporation).
RESULTS
Acute lethal infection model.
The efficacy of AC98-6446 and vancomycin were determined against infections with methicillin-susceptible and -resistant S. aureus, vancomycin-resistant E. faecalis, and penicillin-susceptible and -resistant S. pneumoniae isolates (Table 1). AC98-6446 demonstrated efficacy against both methicillin-susceptible and -resistant S. aureus infections regardless of methicillin susceptibility. ED50s of 0.08 and 0.27 mg/kg were achieved against the methicillin-susceptible S. aureus (MSSA) and MRSA strains, respectively. Vancomycin was approximately 10 times less potent than AC98-6446, with ED50s of 0.69 and 2.89 mg/kg for the MSSA and MRSA infections, respectively. AC98-6446 maintained its excellent efficacy against a VRE infection (ED50, 0.39 mg/kg). Vancomycin, as expected, required a much higher dose to protect mice from infection with the VRE organism. Efficacy of AC98-6446 against the penicillin-susceptible and -resistant S. pneumoniae isolates was comparable, with ED50s of 0.05 to 0.07 mg/kg, respectively. AC98-6446 was 8 to 22 times more effective than vancomycin against these infections. The efficacy of vancomycin decreased between the susceptible and resistant S. pneumoniae infection, demonstrated by an increase in ED50 from 0.4 to 1.55 mg/kg. Overall, AC98-6446 demonstrated excellent in vivo efficacy in this model against all the isolates tested. This data correlates well with previously observed in vitro activity (P. Petersen, P. Labthavikul, T. Wang, R. Dushin, and P. Bradford, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-353, 2002).
TABLE 1.
In vivo efficacy of i.v.-administered AC98-6446 and vancomycin against gram-positive pathogens in a murine acute lethal infection model
| Compoundc | ED50 in mg/kg (95% confidence limitsa)
|
||||
|---|---|---|---|---|---|
| S. aureus (MSSA) | S. aureus (MRSA) | E. faecalis (VRE) | S. pneumoniae (Penrb) | S. pneumoniae (Pensb) | |
| AC98-6446 | 0.08 (0.07-0.11) | 0.27 (0.21-0.34) | 0.39 (0.32-0.48) | 0.05 (0.04-0.07) | 0.07 (0.05-0.09) |
| Vancomycin | 0.69 (0.51-0.97) | 2.89 (1.87-7.0) | 20 (10.8-44.3) | 0.4 (0.31-0.52) | 1.55 (1.27-1.89) |
| LD50d | 1.3 × 105 | 1.1 × 106 | 1.6 × 106 | 1.0 × 101 | 1.8 × 101 |
Seven-day survival ratio from three separate tests, pooled for estimation of ED50 and 95% upper and lower confidence limits by a computerized program for joint probit analysis.
Penr, penicillin-resistant ATCC1894; Pens, penicillin-susceptible ATCC6301.
AC98-6446 MIC (micrograms per milliliter) for S. aureus (MSSA), 0.03; S. aureus (MRSA), 0.015; E. faecalis, 0.06; S. pneumoniae (Penr), <0.008; S. pneumoniae (Pens), <0.008. Vancomycin MIC for S. aureus (MSSA), 1; S. aureus (MRSA), 0.5; E. faecalis, >8; S. pneumoniae (Penr), <0.5; S. pneumoniae (Pens), 0.25.
LD50, 50% lethal dose.
Murine thigh infection model.
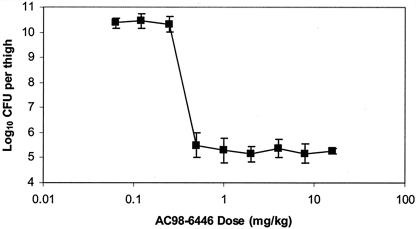
Figure 2 shows the effect of i.v. administration of AC98-6446 to neutropenic CD-1 mice infected intramuscularly with S. aureus Smith. AC98-6446 was administered 1.5 h after infection as a single dose over a range from 0.06 to 16 mg/kg. The bacterial density taken from control animals prior to initiation of dosing was determined to be 7.2 ± 0.35 log10 CFU/thigh, which increased to 11.2 ± 0.78 log10 CFU/thigh after 24 h in the untreated controls. The maximal effect represented by a 2 log10 reduction in counts was observed for doses exceeding 0.5 mg/kg. The dose that resulted in no change of bacterial growth from the initial titer (static dose) was determined to be 0.39 mg/kg. The EC50, or dose resulting in 50% of maximal bacterial killing, was 0.37 mg/kg, with an Emax value of 5.2 CFU/thigh. AC98-6446 exhibited an extreme difference in bactericidal activity between the 0.25 and 0.5 mg/kg doses. The twofold dose level change resulted in a 4.5 log10 difference in bacterial thigh density. The steep dose response between these two doses represents the difference from no effect to maximal effect observed with all doses administered.
FIG. 2.
Bacterial counts in thighs of mice infected with S. aureus Smith. AC98-6446 was administered i.v. as a single dose at 1.5 h postinfection. Bacterial titers were determined 24 h after infection. Mean values ± standard deviation are represented for three mice at each dose level.
Rat endocarditis.
Bacterial vegetation densities were determined in untreated controls at the end of therapy for E. faecalis GC 6181 and at both the initiation and end of therapy for GC 6191. Results are shown in Table 2. The mean bacterial density for untreated control animals (mean log10 numbers of CFU per heart ± the standard deviation) at 4 days after bacterial challenge for GC 6181 was 9.52 ± 0.9. Mean bacterial densities for GC 6191 untreated controls at initiation of therapy and after 4 days were 7.39 ± 0.7 and 9.96 ± 1.2, respectively. Both E. faecalis cardiac infections were treated i.v. with AC98-6446 administered twice a day for total daily doses of 1, 2, 6, and 10 mg/kg. Against E. faecalis GC 6181, a vancomycin-susceptible strain, AC98-6446 demonstrated a dose response reduction in viable bacterial counts compared to counts observed in infected control animals. A dose of 2 mg/kg/day resulted in vegetative counts that were 1.96 log10 lower than those of untreated animals at the end of therapy. Increasing the dose to 6 and 10 mg/kg/day resulted in bacterial reductions of 3.88 and 3.84 log10 CFU, respectively. Total doses of 1, 2, 6, and 10 mg/kg/day of AC98-6446 administered to animals infected with E. faecalis GC 6191 (vancomycin-resistant) resulted in bacterial count reductions of 2.53, 2.29, 4.58, and 5.92 log10 CFU from untreated control titers at the end of therapy, respectively. Compared to the bacterial counts at the initiation of therapy, doses of 6 and 10 mg/kg/day resulted in titer reductions of 2.01 log10 and 3.35 log10, respectively. AC98-6446 therefore demonstrated a bactericidal effect (>3 log10) for the vancomycin-resistant E. faecalis infection when administered at 10 mg/kg/day.
TABLE 2.
Efficacy of AC98-6446 against experimental endocarditis caused by isogenic strains of E. faecalis
| Organism | Treatment | n | Dose (mg/kg/day) | Bacterial titer (log10 CFU/heart)
|
Reduction from control (log10 CFU) | |
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| GC 6181 | None | 22 | 0 | 9.52 | 0.90 | |
| AC98-6446 | 8 | 1.0 | 8.37 | 0.79 | −1.16a | |
| AC98-6446 | 8 | 2.0 | 7.56 | 1.07 | −1.96a | |
| AC98-6446 | 14 | 6.0 | 5.64 | 0.82 | −3.88a | |
| AC98-6446 | 9 | 10.0 | 5.69 | 1.22 | −3.84a | |
| GC 6191 | None | 22 | 0 | 9.96 | 1.28 | |
| AC98-6446 | 12 | 1.0 | 7.43 | 1.12 | −2.53a | |
| AC98-6446 | 9 | 2.0 | 7.68 | 1.12 | −2.29a | |
| AC98-6446 | 8 | 6.0 | 5.38 | 0.92 | −4.58a | |
| AC98-6446 | 9 | 10.0 | 4.04 | 0.78 | −5.92a | |
Statistically significant difference (P < 0.001) as determined by t test.
Pharmacokinetics.
The pharmacokinetics of i.v.-administered AC98-6446 in four species are shown in Table 3. The maximum observed concentration of AC98-6446 after 20-mg/kg i.v. administration to mice, rats, monkeys, and dogs was 31.6, 231, 188, and 201 μg/ml, respectively. The compound demonstrates increased exposure as measured by the area under the concentration curve with higher species. The area under the concentration curve increases from 164 μg · h/ml in mice to 241 μg · h/ml in rats, 860 μg · h/ml in monkeys, and 1,247 μg · h/ml in dogs. The observed increased exposure corresponds to a decreased clearance of the compound from the plasma in the higher species. Clearance values (in milliliters per hour per kilogram) decrease from 121 and 83 in the mouse and rat to 23 and 16 in the monkey and dog. The plasma t1/2 also reflects this with values of 3.3, 1.5, 11.5, and 11 h observed for the mouse, rat, monkey, and dog, respectively.
TABLE 3.
Pharmacokinetic parameters for AC98-6446 following i.v. administration of a single 20-mg/kg dose to mice, rats, monkeys, and dogs
| Animal | Cmax (μg/ml) | AUC (μg/h/ml) | t1/2 (h) | CL (ml/h/kg) | Vss (liters/kg) |
|---|---|---|---|---|---|
| Mouse | 316 | 164 | 3.3 | 121 | 1.8 |
| Rat | 231 | 241 | 1.5 | 83 | 0.21 |
| Monkey | 188 | 860 | 11.5 | 23 | 0.2 |
| Dog | 201 | 1,247 | 11 | 16 | 0.3 |
Cmax, maximal concentration observed; AUC, area under the concentration curve; t1/2, plasma half-life; CL, clearance; Vss, volume of distribution at steady state.
DISCUSSION
The increase in the number of gram-positive bacteria with multidrug resistance occurring in the last several years has resulted in infections that are difficult to treat. This is of particular concern regarding staphylococci and enterococci. The incidence of MRSA has risen to be the cause of up to 21% of skin infections and 59% of nosocomial pneumonia in certain areas (20). Infections with MRSA have become a concern in long-term care facilities from patients colonized with MRSA in hospitals prior to transfer to the facility (15). Until recently, vancomycin has been the drug of choice for these infections (24). However, the emergence of S. aureus with reduced susceptibility to vancomycin has been reported from the United States, France, Spain, United Kingdom, Japan, Korea, and China (29). These isolates, with vancomycin MICs ranging from 4 to 8 μg/ml, have been referred to as VISA, for vancomycin-intermediate S. aureus (13). Many of these isolates exhibiting reduced susceptibility to the glycopeptides have been associated with therapeutic failures with vancomycin (29). Resistance has been reported to be associated with increased cell wall synthesis and a thickened or aggregated cell wall (23). Infections with these isolates represents a therapeutic challenge.
The enterococci have emerged as an increasingly important pathogen because of acquired resistance to the glycopeptides (VRE) and other agents. Glycopeptide resistance rates vary from extremely low for E. faecalis in the Asia Pacific and Latin America to over 50% for E. faecium in North America (17). VRE colonization appears to be more frequent than actual infection (11) and predominates in the intensive care unit, with nosocomial transmission a particular concern (19, 21). Resistance rates for vancomycin and some of the newer agents underscore the need for alternative therapy (10, 18, 27).
AC98-6446 is a semisynthetic derivative belonging to the mannopeptimycin family of glycopeptide antibiotics that selectively targets bacterial cell wall synthesis (25). It has been shown to be a potent bactericidal inhibitor of resistant gram-positive bacteria, including methicillin-resistant and glycopeptide-intermediate S. aureus as well as vancomycin-resistant E. faecalis and E. faecium (Petersen et al., 42nd ICAAC). The in vitro activity observed in these studies was also exhibited by in vivo efficacy.
Our results indicate that AC98-6446 was more effective than vancomycin against infections with MSSA and MRSA, vancomycin-resistant E. faecalis, and penicillin-susceptible and -resistant S. pneumoniae. Efficacy of AC98-6446 was not dependent on the resistance phenotype of the organism. We observed equivalent efficacy regardless of methicillin resistance in S. aureus or penicillin resistance in S. pneumoniae. Of particular note is that AC98-6446 was over 10 and 50 times more efficacious than vancomycin against infections with the MRSA and VRE strains, respectively.
As previously reported, AC98-6446 exhibited bactericidal activity against staphylococci, streptococci, and enterococci as well as a long postantibiotic effect (Petersen et al., 42nd ICAAC). The results of the thigh infection model from this study show a sharp decline in bacterial counts in infected thighs within a twofold dose range. Increasing the dose administered from 0.25 to 0.5 mg/kg resulted in the difference between a 3 log10 increase and a 1.5 log10 decrease from the initial infection level. Increasing doses up to 16 mg/kg did not affect the observed bactericidal activity of the compound. Further pharmacodynamic studies are required to assess the implications of this and the parameters required for efficacy of the compound.
Infective endocarditis due to complications from intravenous drug use, prosthetic valves, and nosocomial bacteremia results in extended hospital stays and high mortality rates (26). This is especially true when infection is due to VRE or MRSA (4). AC98-6446 was clearly effective in preventing growth of both vancomycin-susceptible and vancomycin-resistant E. faecalis isolates in an infective endocarditis model. It exhibited greater efficacy at a lower dose than was previously reported for vancomycin against these same strains (1.5 and 0.14 log10 reduction against GC 6181 and GC 6191, respectively, at a dose of 40 mg/kg/day) (16). In addition, bactericidal activity (>3 log10 reduction in viable counts) was observed against the VRE strain from counts at the initiation of therapy. These results underscore the excellent activity of AC98-6446 against VRE in addition to its ability to treat this difficult deep-seated infection.
The infection models described in this study involved the use of rodent species (mouse and rat) only. Pharmacokinetic exposure of AC98-6446 was moderate in the mouse and rat, with t1/2 of 3.3 and 1.5 h and plasma clearance of 121 and 83 ml/h/kg. Higher species (monkey and dog) exhibited longer plasma exposure and slower clearance values. Efficacy, if related to overall exposure, could therefore require a lower administered dose in higher species.
Overall, AC98-6446, representative of a novel class of glycopeptide antibiotics, exhibits excellent in vitro activity against resistant gram-positive pathogens, which was also demonstrated in vivo in three different animal models of infection. Efficacy against infections involving MRSA and vancomycin-resistant E. faecalis and an acceptable pharmacokinetic profile coupled with a unique mechanism of action (A. Ruzin, G. Singh, A. Severin, Y. Yang, R. Dushin, A. Sutherland, A. Minnick, M. Greenstein, M. May, D. Shlaes, and P. Bradford, unpublished results) make this compound an excellent candidate for further study.
Acknowledgments
We greatly appreciate and recognize the contributions of Pete Petersen, Russell Dushin, Patricia Bradford, Raymond Testa, Steve Projan, John O'Connell, David Shlaes, and Tarek Mansour.
REFERENCES
- 1.Calderon, E., J. Arredo-Garcia, F. Aguilar, and P. Garcia-Roca. 2003. In vitro antimicrobial susceptibility in clinical isolates of Enterococcus species. Salud Publica de Mexico 45:96-101. [DOI] [PubMed] [Google Scholar]
- 2.Chavers, L., S. Moser, W. Benjamin, S. Banks, J. Steinhauer, A. Smith, C. Johnson, E. Funkhouser, L. Chavers, A. Stamm, and K. Waites. 2003. Vancomycin-resistant Enterococci: 15 years and counting. J. Hosp. Infect. 53:159-171. [DOI] [PubMed] [Google Scholar]
- 3.Denis, O., C. Nonhoff, B. Byl, C. Knoop, S. Bobin, and M. Struelens. 2002. Emergence of vancomycin-intermediate Staphylococcus aureus in a Belgian hospital. J. Antimicrob. Chemother. 50:383-391. [DOI] [PubMed] [Google Scholar]
- 4.Eliopoulos, G. 1992. Enterococcal endocarditis, p. 209-223. In D. Kaye (ed.), Infective endocarditis, 2nd ed. Raven Press Ltd., New York, N.Y.
- 5.Finney, D. 1971. Probit analysis, 3rd ed. Cambridge University Press, London, United Kingdom.
- 6.Fluit, A., C. Wielders, J. Verhoef, and F. Schmitz. 2001. Epidemiology and susceptibility of 3,051 Staphylococcus aureus isolates from 25 university hospitals participating in the European SENTRY study. J. Clin. Microbiol. 39:3727-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grohskopf, L., R. Sinkowitz, D. Garrett, A. Sohn, G. Levine, J. Siegel, B. Stover, and W. Jarvis. 2002. A national point-prevalence survey of pediatric intensive care unit-acquired infections in the United States. J. Pediatr. 140:432-438. [DOI] [PubMed] [Google Scholar]
- 8.He, H., T. Williamson, B. Shen, E. Graziani, H. Yang, S. Sakya, P. Petersen, and G. Carter. 2002. Mannopeptimycins, novel antibacterial glycopeptides from Streptomyces hygroscopicus, LL-AC98. J. Am. Chem. Soc. 124:9729-9736. [DOI] [PubMed] [Google Scholar]
- 9.Joels, C., B. Matthews, L. Sigmon, R. Hansan, C. Lohr, K. Kercher, J. Norton, R. Sing, and B. Heniford. 2003. Clinical characteristics and outcomes of surgical patients with vancomycin-resistant enterococcal infections. Am. Surg. 69:514-519. [PubMed] [Google Scholar]
- 10.Johnson, A., M. Warner, G. Hallas, and D. Livermore. 2000. Susceptibility to quinupristin/dalfopristin and other antibiotics of vancomycin-resistant enterococci from the UK, 1997 to mid-1999. J. Antimicrob. Chemother. 46:125-128. [DOI] [PubMed] [Google Scholar]
- 11.Lai, K., S. Fontecchio, A. Kelly, S. Baker, and Z. Melvin. 2003. The changing epidemiology of vancomycin-resistant enterococci. Infect. Control Hosp. Epidemiol. 24:264-268. [DOI] [PubMed] [Google Scholar]
- 12.Lepelletier, D., and H. Richet. 2001. Surveillance and control of methicillin-resistant Staphylococcus aureus infections in French hospitals. Infect. Control Hosp. Epidemiol. 22:677-682. [DOI] [PubMed] [Google Scholar]
- 13.Linares, J. 2001. The VISA/GISA problem: therapeutic implications. Clin. Microbiol. Infect. 7(Suppl. 4):8-15. [DOI] [PubMed] [Google Scholar]
- 14.Livermore, D. 2000. Antibiotic resistance in staphylococci. Int. J. Antimicrob. Agents 16(Suppl. 1):S3-S10. [DOI] [PubMed] [Google Scholar]
- 15.Mendelson, G., Y. Yearmack, E. Granot, J. Ben-Israel, R. Colodner, and R. Raz. 2003. Staphylococcus aureus carrier state among elderly residents of a long term care facility. J. Med. Direct. Assoc. 4:125-127. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, T., J. Deitz, P. Petersen, S. Mikels, and W. Weiss. 2000. Therapeutic efficacy of GAR-936, a novel glycylcycline, in a rat model of experimental endocarditis. Antimicrob. Agents Chemother. 44:3022-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutnick, A., D. Biedenbach, and R. Jones. 2003. Geographic variations and trends in antimicrobial resistance among Enterococcus faecalis and Enterococcus faecium in the SENTRY Program. Diagn. Micrbiol. Infect. Dis. 46:63-68. [DOI] [PubMed] [Google Scholar]
- 18.Nilius, A. 2003. Have the oxazolidinones lived up to their billing? Future perspectives for this antibacterial class. Curr. Opin. Investig. Drugs 4:149-155. [PubMed] [Google Scholar]
- 19.Pacio, G., P. Visintainer, G. Maguire, G. Wormser, J. Raffalli, and M. Montecalvo. 2003. Natural history of colonization with vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus and resistant gram-positve bacilli among long term care facility residents. Infect. Control Hosp. Epidemiol. 24:246-250. [DOI] [PubMed] [Google Scholar]
- 20.Pechere, J. 1999. Current and future management of infections due to methicillin-resistant staphylococci infections: the role of quinupristin/dalfopristin. J. Antimicrob. Chemother. 44(Suppl. A):11-18. [DOI] [PubMed] [Google Scholar]
- 21.Rahim, S., S. Pillai, H. Gold, L. Venkataraman, K. Inglima, and R. Press. 2003. Linezolid-resistant, vancomycin-resistant Enterococcus faecium infection in patients without prior exposure to linezolid. Clin. Infect. Dis. 36:146-148. [DOI] [PubMed] [Google Scholar]
- 22.Rennie, R., R. Jones, and A. Mutnick. 2003. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn. Microbiol. Infect. Dis. 45:287-293. [DOI] [PubMed] [Google Scholar]
- 23.Rybak, M., and R. Akins. 2001. Emergence of methicillin-resistant Staphylococcus aureus with intermediate glycopeptide resistance: clinical significance and treatment options. Drugs 61:1-7. [DOI] [PubMed] [Google Scholar]
- 24.Sieradzki, K., T. Leski, J. Dick, L. Borio, and A. Tomasz. 2003. Evolution of a vancomycin-intermediate Staphylococcus aureus strain in vivo: multiple changes in the antibiotic resistance phenotypes of a single lineage of methicillin-resistant S. aureus under the impact of antibiotics administered for chemotherapy. J. Clin. Microbiol. 41:1687-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh, M., P. Petersen, W. Weiss, J. Janso, S. Luckman, E. Lenoy, P. Bradford, R. Testa, and M. Greenstein. 2003. Mannopeptimycins, new cyclic glycopeptide antibiotics produced by Streptomyces hygoscopicus LL-AC98: antibacterial and mechanistic activities. Antimicrob. Agents Chemother. 47:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobel, J. 1992. Nosocomial infective endocarditis, p. 316. In D. Kaye (ed.), Infective endocarditis, 2nd ed. Raven Press Ltd., New York, N.Y.
- 27.Strahilevitz, J., and E. Rubenstein. 2002. Novel agents for resistant gram-positive infections-a review. Inter. J. Infect. Dis. 6(Suppl. 1):S38-S46. [DOI] [PubMed] [Google Scholar]
- 28.Sum, P., D. How, N. Torres, P. Petersen, E. Lenoy, W. Weiss, and T. Mansour. 2003. Novel ether derivatives of mannopeptimycin glycopeptide antibiotic. Bioorg. Med. Chem. Lett. 13:1151-1155. [DOI] [PubMed] [Google Scholar]
- 29.Tenover, F. 1999. Implications of vancomycin-resistant Staphylococcus aureus. J. Hosp. Infect. 43(Suppl.):S3-S7. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Y., S. Geng, F. Lai, and N. Wang. 2002. Survey of drug resistance to ciprofloxacin in common pathogenic bacteria. Di Yi Jun Daxue Xuebao 22:373-379. [PubMed] [Google Scholar]