Figure 4. BC groove specificity for BH3-only ligands.
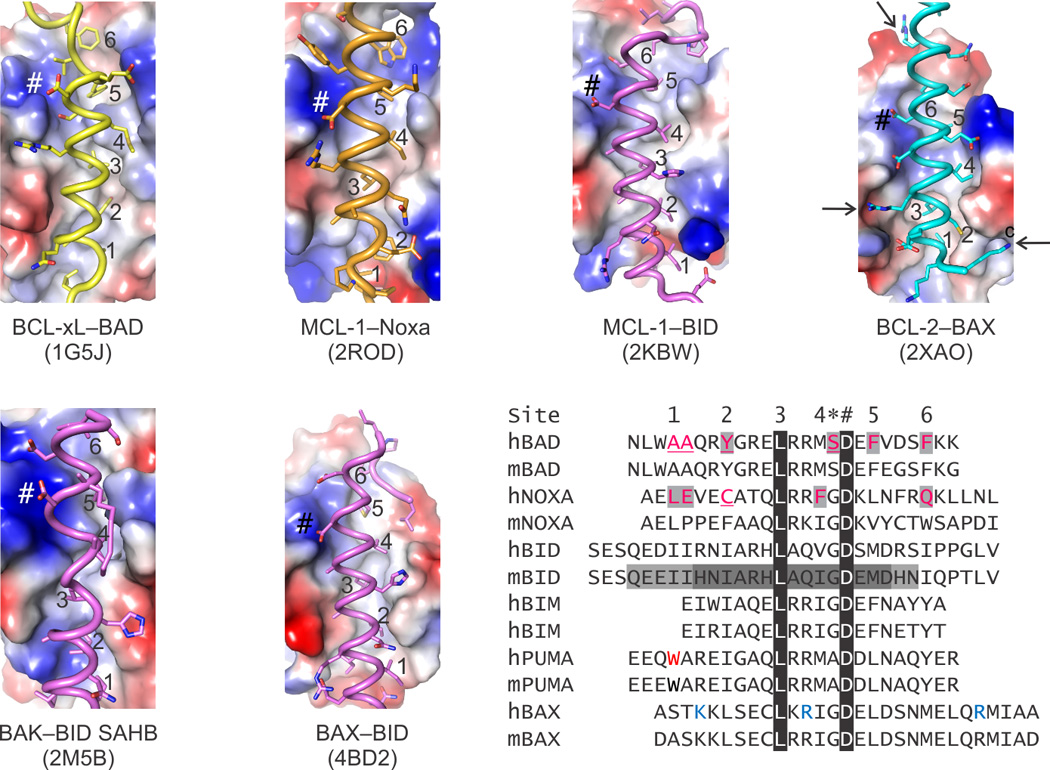
The electrostatic potential on the surface of BC grooves reveals the similarities and differences between folded BCL-2 family proteins, which ultimately dictate the specificity of the binding pairs as summarized in Box 1. Sequence homology highlights features shared by BH3 ligands, including up to seven hydrophobic residues that engage six pockets along the BC groove (labeled 1–6 in the sequence alignment and in the structures) and a conserved Asp always found in a salt bridge (labeled *) preceded by a small residue (labeled #). Peripheral to these positions, which are marked in each structure panel, there are additional interactions, many electrostatic in nature, that contribute to the specificity. For instance, in the BCL-2–BAX complex three positively charged Lys and Arg residues (blue in the alignment and identified by arrows in the structure) were shown to be essential for the affinity of BAX for BCL-2. In addition to the specificity, the BC grooves have evolved to be selective only for certain BH3s; others typically produce structural clashes when modeled based on the known complexes. Up to four residues in BAD BH3 and Noxa BH3, predicted to clash, had to be replaced to directly activate BAK and BAX. Residues highlighted and underlined in human BAD and NOXA were replaced by those found in human BID BH3 for BAK and BAX activation, respectively. Interestingly, specificity of BID BH3 for BAK and BAX was conferred by minimal BH3 regions that required, respectively, a C- and N-terminal extension to a common core (sequence highlighted by dark gray). Black highlights show 100% sequence conservation. Electropositive, electronegative, and neutral (hydrophobic) patches are blue, red and white, respectively.
