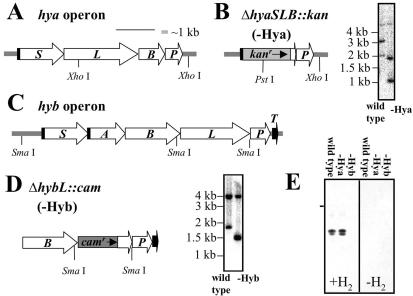
FIG. 1.
Structures and mutagenesis of putative hya and hyb operons. (A) Structure of putative hya operon. Twin-arginine motifs are indicated as vertical black bars. (B) Structure of disrupted hya operon and confirmation of Hya-deficient mutant genotype by Southern blotting. Wild-type and Hya-deficient G. sulfurreducens genomic DNAs were cleaved with PstI and XhoI, blotted, and probed with the 2.4-kb DNA fragment used to introduce the ΔhyaSLB::kan mutation into the genome (see Materials and Methods). Expected radiolabeled bands are 5.28, 3.03, and 2.04 kb for the wild-type strain and 5.28, 1.88, and 1.02 kb for the mutant. (C) Structure of putative hyb operon. Twin-arginine motif sequences are indicated as vertical black bars. (D) Structure of disrupted hyb operon and confirmation of Hyb-deficient mutant genotype by Southern blotting. Wild-type and Hyb-deficient G. sulfurreducens genomic DNAs were cleaved with SmaI, blotted, and probed with the 2.0-kb DNA fragment used to introduce the ΔhybL::cam mutation into the genome (see Materials and Methods). Expected radiolabeled bands are 4.02 and 1.76 kb for the wild-type strain and 4.02 and 1.41 kb for the mutant. (E) In-gel hydrogenase activity of Triton X-114 extracts of membranes prepared from acetate-fumarate cultures of the wild-type and Hya- and Hyb-deficient strains. Nondenaturing polyacrylamide gels were stained for hydrogen-dependent benzyl viologen reductase activity as described in Materials and Methods. Twenty micrograms of solubilized membranes was loaded per lane. Lanes 1 to 3 were incubated under hydrogen, while lanes 4 to 6 were incubated under nitrogen. The horizontal bar indicates the interface of the stacking and resolving gels.