Abstract
Dopamine (DA) is known to be the most potent activator of tick salivary secretion, which is an essential component of successful tick feeding. We examined the quantitative changes of catecholamines using a method coupling high-pressure liquid chromatography with electrochemical detection (HPLC-ECD). We also investigated the levels of catecholamines conjugated to other molecules utilising appropriate methods to hydrolyse the conjugates. Three different biological samples, salivary glands, synganglia, ovaries and haemolymph were compared, and the largest quantity of DA was detected in salivary gland extracts (up to ∼100 pg/tick), supporting the hypothesis that autocrine/paracrine dopamine activates salivary secretion. Quantitative changes of catecholamines in the salivary glands over the entire blood feeding duration were examined. The amount of dopamine in the salivary glands increased until the day 5 of feeding, at which the rapid engorgement phase began. We also detected a small but significant amount of norepinephrine in the salivary glands. Interestingly, saliva collected after induction of salivary secretion by the cholinergic agonist pilocarpine contained a large amount of DA sulphate with a trace amount of DA, suggesting a potential biological role of DA sulphate in tick saliva.
Keywords: catecholamine, dopamine purification, salivary secretion, dopamine sulfate, hydrolysis
1. Introduction
Dopamine (DA) and other catecholamines (CA) play major roles in neurophysiological systems as neurotransmitters and hormones in Eukaryotes. In arthropods, in addition to its neural function, DA also functions as a structural component for cross-linking the cuticle, resulting in sclerotisation of the exoskeleton (Arakane et al., 2009; Gorman and Arakane, 2010; Kerkut, 1973). In haematophagous ticks, one important known function of DA is the activation of salivary secretion, which is essential for successful feeding on the host. DA also functions in plasticisation of the cuticle upon increasing body volume during the rapid engorging phase (Kaufman et al, 2010).
The salivary glands (SG) of ticks, especially those of female hard ticks, have been extensively studied to identify their bioactive components and potential antigens of vaccine development (Anisuzzaman et al., 2012; Bowman and Sauer, 2004; Das et al., 2001; Decrem et al, 2008; Jaworski et al, 1992; Mulenga et al, 1999; Prevot et al, 2007; Trimnell et al, 2005; Xu et al, 2005). The components of salivary secretion include cement to attach and seal the mouthparts to the host, as well as other components with anti-haemostatic, immunosuppressive, and anti-inflammatory activities to facilitate blood feeding (Anguita et al, 2002; Francischetti et al, 2002; Ribeiro et al, 1985; Valenzuela et al, 2000). SG are also major excretory organs during feeding. During the rapid engorging phase of feeding, excessive water and ions are excreted through the SG into the host (Kaufman and Phillips, 1973). Changes in the salivary components throughout the tick feeding phases, which span over a week, have also been indicated via 2D gel analyses of salivary glands (Narasimhan et al., 2007). Based on the fact that the SG consist of at least three different types of acini (type 1, 2, and 3) and numerous heterogeneous cells with temporal dynamics, they are likely regulated by a complex mechanism controlling the secretion of salivary components.
Mechanisms controlling tick salivary glands have long been studied primarily using pharmacological tools. The most potent pharmacological stimulator of tick salivary secretion, DA, has been considered as the endogenous signal regulating the SG (Kaufman, 1976; Kaufman, 1977; McSwain et al, 1992; Schmidt et al, 1982). Other in vitro and in vivo pharmacological stimulators, such as norepinephrine and ergot alkaloids, are less active than DA and likely act through the dopamine receptors based on the activities of these chemicals on the recombinant tick dopamine D1 receptor (Simo et al, 2011). Another pharmacological stimulator, pilocarpine (PC), is considered to be acting through cholinergic receptor which activates the neural circuit for salivary secretion in the synganglion (brain of the tick) because it exhibits activity only when the intact synganglion is attached to the SG (Kaufman, 1978; Kaufman and Harris, 1983). In addition, we have demonstrated that at least two neuropeptides, myoinhibitory peptide and SIFamide, are involved in regulation of the SG (Simo et al, 2009a; Simo et al, 2009b). The peptidergic neuronal projections from the synganglion reach the basal region of acini types 2 and 3, and their receptors are also expressed in the SG (Simo et al, 2012; Simo et al, 2013a).
The source of DA for activation of tick salivary secretion has been elusive until recently, however. Whereas activation of SG through dopaminergic neurons has been a general assumption (Bowman and Sauer, 2004), finding more than one nanogram of DA per pair of SG in various partially fed phases of the Amblyomma hebraeum tick led to a hypothesis that autocrine or paracrine DA acts on the SG (Kaufman et al, 1999). In another recent study, DA immunohistochemistry revealed immunoreactive granules in the basal cells of acini in the SG only during the early feeding phase, from 24 to 48 hours after the onset of feeding, in a different species of hard tick, Ixodes scapularis (Simo et al, 2011). The disparity regarding the timing of DA production in two different Ixodid tick species, using two different detection methods, from previous studies warrants further investigation.
In light of previous studies and general lack of information on DA in tick, we examined quantities of DA in different biological samples (tissues) and during various feeding stages in the female Ixodes scapularis and investigated the quantities of the metabolic derivatives of CA, DA, dopamine sulphates and other catecholamine conjugates in the SG throughout the feeding phases. In addition, we investigated whether DA was included in tick salivary secretion for modulation of host response.
2. Materials and methods
2.1. Chemicals
Citric acid monohydrate, anhydrous sodium acetate, sulphatase from Aerobacter aerogenes, pilocarpine and CA standards, such as norepinephrine, DA and isoproterenol (ISO), were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade methanol was from EMD Millipore (Billerica, MA, USA). Ethylenediamine tetra-acetic acid disodium salt (EDTA.2Na) and sodium 1-octanesulphonate were from Dojindo Laboratories (Kumamoto, Japan). DA-3-sulphate, and DA-4-sulphate was obtained from the NIMH Chemical Synthesis and Drug Supply Program, RTI International.
2.2. Feeding and sampling of ticks
Adult I. scapularis ticks were obtained from a tick rearing facility at Oklahoma State University. Ticks were fed on rabbits in rubber capsules (∼5 × 5 cm) glued onto the shaved backs of the animals using non-irritant Latex glue covered with a fine mesh net (Koci et al., 2013). For SG sampling, female ticks were collected every day until full engorgement after initiation of feeding on a naïve rabbit. Thus, the samples consisted of unfed ticks (D0), ticks feeding from one to six days (D1-D6) and fully engorged (FE) ticks collected at the seventh day of feeding. Samples were collected at 24-hour intervals, such that D1 is corresponds to a sample collected 0 to 24 hours after the onset of feeding. For sampling of different tissues in the DA recovery/depletion assay, female ticks were removed on D5. For the collections of saliva, D1 and D5 ticks were sampled in order to investigate and compare saliva of ticks from slow and fast feeding phase. Three biological replicates were performed, all using naïve rabbits. The animal protocol was approved by the Kansas State University Institutional Animal Care and Use Committee (IACUC) to minimise the suffering of the rabbits.
2.3. Tick saliva collection
A five microliter-capillary tube was placed on the hypostome of each washed and immobilised tick. The ticks immobilised on dental wax were injected with pilocarpine in Hank's solution (10 mg/ml) through the dorsal posterior region (∼0.5 μL for D1 ticks and ∼3 μL for D5 ticks) and were placed in a humidified chamber at 37 °C for 2 hours. The saliva samples collected were stored at -80 °C until use. The quantities of tick saliva collected using this method varied largely, from 0 to 40 μL per individual, depending on the individual and the day of feeding.
2.4. Sample preparation for CA analysis
The tick organs, SG, synganglia, and ovaries were dissected in ice-cold PBS, pH 7.2, within 5 min after removal from the rabbit in order to avoid any artefacts introduced between post-detachment and dissection. For extraction of CA and their metabolic derivatives, we used three different extraction methods (Fig. 1). The tissue homogenate was used to extract free CA, which was purified using aluminium oxide (CA-free). The homogenate after removal of CA-free was divided in half; one half of the homogenate was treated with sulphatase and used for aluminium oxide-purification of CA, henceforth referred to as CA sulphates (CA-S); the remaining half was hydrolysed and used for aluminium oxide -purification of CA, henceforth referred to as CA conjugated with other compounds (CA-X).
Figure 1.
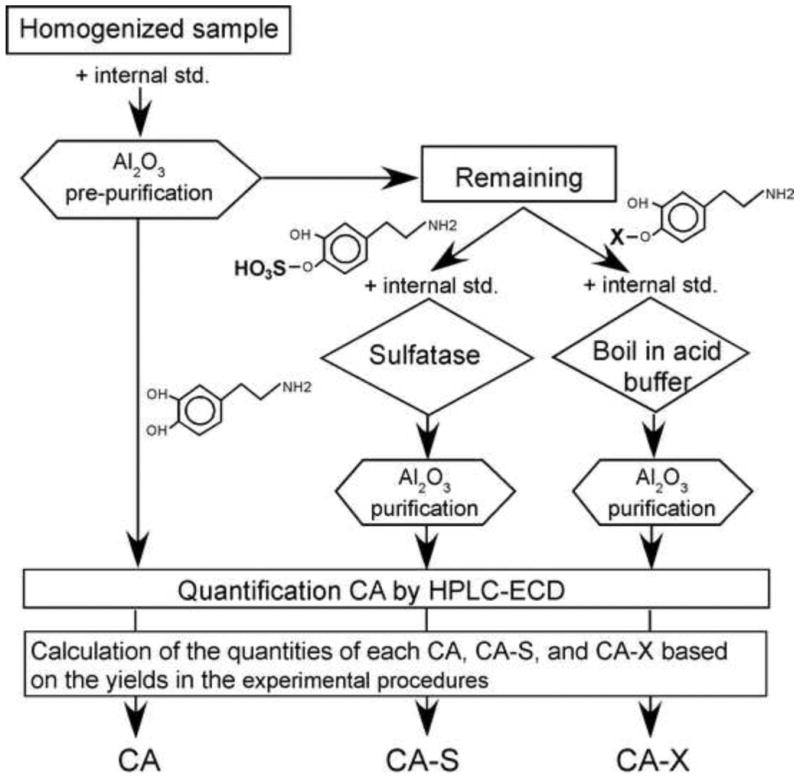
Diagram illustrating the experimental procedure for quantification of catecholamines (CA), catecholamine-sulphates (CA-S), and other conjugated catecholamines (CA-X). See materials and methods for details.
For aluminium oxide pre-purification of free CA, each tissue sample was homogenised using a plastic pestle in 100 μl of 1 M Trizma-HCl, pH 9, which contained 1 ng of the internal standard ISO for a single tick equivalent tissue. After homogenisation, 200 μl of 1 M Trizma-HCl was added with 30 μl of 0.1 M EDTA.2Na and ∼30 mg of aluminium oxide. The mixture was incubated at RT in a rotary shaker for 15 min and centrifuged at 1000×g for 1 min. Aluminium oxide was washed three times with distilled deionised water (ddH2O) and dehydrated in a PVDF filter column (5000×g for 10 min, 4 °C). CA were eluted using 10% acetic acid with 100 μM EDTA.2Na after incubation for 15 min on ice and were centrifuged at 5000×g for 10 min, 4 °C. Twenty microliters of elution buffer were used per single tick equivalent organs.
One half of the supernatant after aluminium oxide absorption described above was used for CA-S extraction. The supernatant was mixed with 95 mU of sulphatase (Aerobacter aerogenes) and 1 ng of ISO per single tick equivalent, then incubated at 37 °C for 20 min. This procedure hydrolysed ∼80% of both DA-3-sulphate and dopamine-4-sulphate (data not shown), which was used as a correction factor for calculation of CA-sulphate quantity. After sulphatase incubation, 300 μl of 1 M Trizma-HCl, pH 9, 30 μl of 0.1 M EDTA.2Na, and ∼30 mg of aluminium oxide were added, and the same extraction protocol as that of CA-free was performed.
The remaining half of the supernatant after the CA purification was used for CA-X extraction. The supernatant was mixed with 1/10 volume of 10 M HCl, 1/50 volume of Na2S2O5 and 1 ng of ISO per single tick equivalent. The mixture was boiled in a water bath for 30 min, placed on ice for 10 min and mixed with 300 μl of 1 M Trizma-HCl, pH 9, 30 μl of 0.1 M EDTA.2Na, and 30 mg of aluminium oxide. The extraction protocol was the same as that of DA-free. In the subsequent analyses, the final quantities of CA and CA-S represented the normalised values for each tick, while CA-X quantity was calculated and expressed as the quantity of the total hydrolysed CA subtracted by that of CA-S for each tick. The proportions of DA, DA-X, and DA-S were calculated by dividing each component by the total quantity.
2.5. High-pressure liquid chromatography and electrochemical detection (HPLC-ECD)
HPLC analyses were performed using a HTEC-500 (Eicom Corp., Kyoto, Japan), standalone HPLC system incorporating an electrochemical detector consisting of a counter electrode, a pure graphite WE-PG working electrode and an Ag/AgCl RE-100 reference electrode. Separation was performed using a SC-50DS HPLC column, 3 mm × 150 mm (Eicom Corp.), with a pre-column packed with AC-0DS packing matrix (Eicom Corp.). Analyses of CA were performed by injecting 20-μl aliquots, one tick equivalent, for each sample. The flow rate was 500 μl/min with a mobile phase solution containing citric acid (8.4 g/L), sodium acetate (3.1), sodium 1-octanesulphonate (0.22), EDTA.2Na (0.05) and methanol (15%), pH 3.5. The effluent was monitored electrochemically at a potential of +500 mV versus Ag/AgCl. Quantification of CA was performed using PowerChrom software (eDAQ Pty Ltd., Deniston East, Australia) with standard curves and the peak area method. In addition, ISO served as an internal standard to compensate for variations in the recovery rate during the purification process.
2.6. Effect of pilocarpine on DA quantity
Ticks were washed in ddH2O and 70% ethanol and immobilised onto a Petri dish using dental wax. Either Hank's solution alone as a control or 10 mg/ml pilocarpine in Hank's solution (10 μl for D1 ticks and 50 μl for D5 ticks) was applied to the tick dorsum and housed in a humidified chamber at 37 °C. Samples were obtained at two time points, 30 min and 4 hours. Two ticks per group, washed in ddH2O, were used for collections of haemolymph, SG and synganglia. Samples were stored at -80 °C until extraction of free and hydrolysed DA.
Summary of the biological material used in this study including the sample numbers can be found in table 1 of supplementary material.
3. Results
3.1. CA purification, detection, and quantification
The yields obtained after pre-purification of DA were 86.8±10.4, 72.6±16.5, and 72.6±16.2% for free DA extraction, hydrolysis, and sulphatase treatment procedures, respectively, indicating mild to moderate degrees of loss in the pre-purification steps. Therefore, subsequent quantification included the value after compensating for losses of yield using the internal control ISO.
Our HPLC-ECD protocol routinely detected the standard chemicals norepinephrine, 3,4-dihydroxylbenzylamine, DA, and serotonin (ordered by their elution time), while detection of octopamine and tyramine required higher detection sensitivity, and L-DOPA (L-3,4-dihydroxyphenylalanine) was masked by early elution peaks in this protocol. Based on standard curves, the detection limit for DA, NE and ISO was 1 pg (Fig. 1 in supplementary material), which was sufficient for CA calculation in most of the single tick equivalent samples.
3.2. Spatial and temporal changes in CA quantity
Tissue-specific quantification of DA, DA-X, and DA-S revealed the presence of the highest quantities of all three forms in the SG, among which the most abundant form was DA-S (157.8 pg/tick SG), followed by DA (94.1 pg/tick SG) and DA-X (91.5 pg/tick SG). DA in the SG was 7.5-fold and 960-fold more abundant than in synganglia (SYN) and ovaries (OVA), respectively (Fig. 2A).
Figure 2.
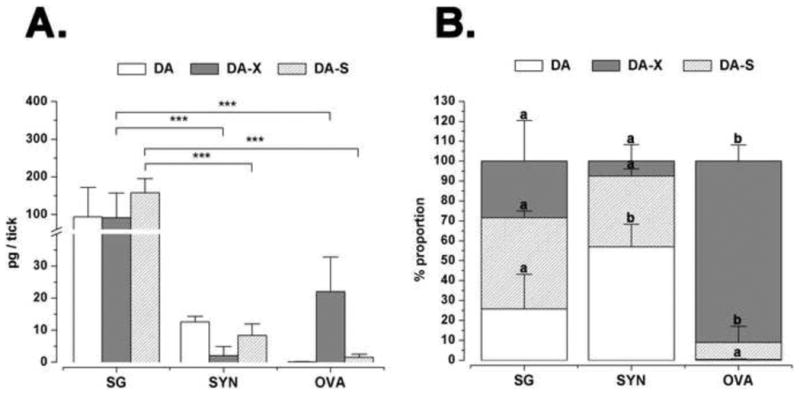
(A) Tissue-specific quantification of dopamine and its conjugates in the D5 tick salivary glands (SG), synganglia (SYN) and ovaries (OVA). Procedures for measuring the quantities of free dopamine (DA), conjugated dopamine (DA-X), and dopamine sulphate (DA-S) are in the text. (B) The data represent the proportions of dopamine and its conjugates in each tick organ. Values in the chart represent means of three biological replicates with error bars representing standard deviation (S.D.). In A and B, asterisks and letters indicate significant differences of p<0.001 and p<0.05, respectively, based on one-way ANOVA with Tukey post-hoc comparison.
Analysis of the temporal changes in DA, DA-X, and DA-S quantity from unfed (D0) to fully engorged (FE) ticks sampled on D7 (Fig. 3, 4A) revealed that the amount of DA gradually increased from 18.8 in unfed to 94.1 pg/tick SG on D5, followed by decreases to 65.2 pg in D6 and 39.6 pg in FE ticks. DA-X and DA-S displayed similar patterns in the course of feeding, except for a relatively high DA-S quantity in unfed ticks. Both DA-X and DA-S declined on the first day of feeding (6.2 pg of DA-X and 22.8 pg of DA-S), followed by gradual increases to their peaks on D6 (241.3 pg and 198.7 pg of DA-X and DA-S, respectively), then declined in the fully engorged ticks, of which the SG contained 68.3 pg of DA-X and 178.3 pg of DA-S (Fig. 4A).
Figure 3.
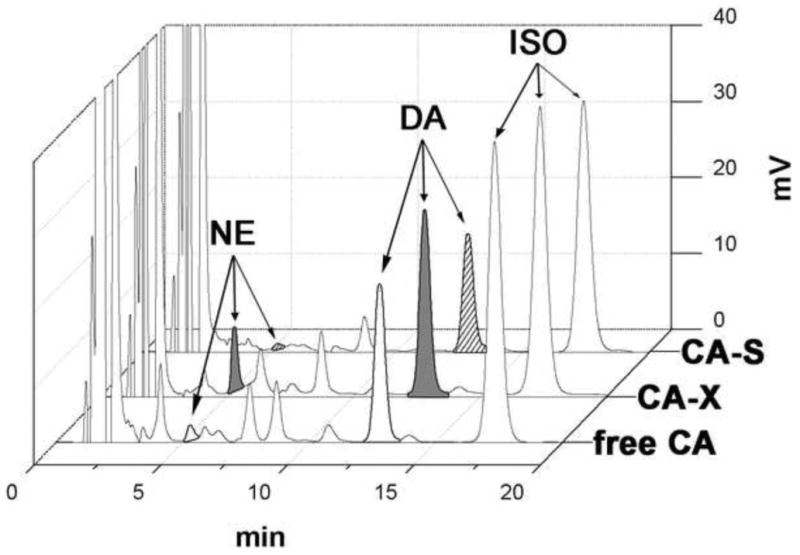
HPLC-ECD chromatograms of the free catecholamines (free CA), hydrolysed catecholamines (CA-X) and sulphohydrolysed catecholamines (CA-S) extracted from salivary glands of 5 day-fed female I. scapularis tick. Each chromatogram represents injection of a single tick equivalent.
Figure 4.
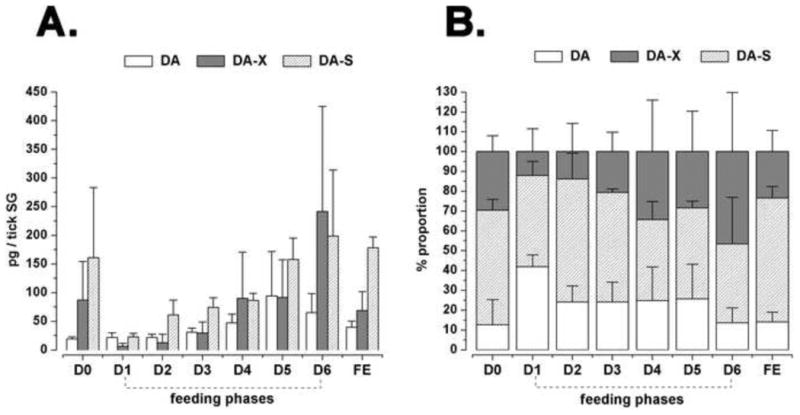
(A) Feeding phase-specific quantification of dopamine and its conjugates in tick salivary glands. (B) Data are also provided representing the proportions of dopamine and its conjugates. The feeding phases are unfed (D0), daily feeding samples (D1 to D6), and fully engorged (FE) on the seventh day. Procedures for measuring the quantities of free dopamine (DA), conjugated dopamine (DA-X), and dopamine sulphate (DA-S) are in the text. The data represent the mean of three biological replicates with standard deviation (S.D.).
The proportion of DA was lowest in unfed ticks (12.6%) and highest on the first day of feeding (42%, Fig. 4B). Between days 2-5, the percent of free DA fluctuated in a range of 24.1 to 25.7%, and declined to 13.6% and 14% on day 6 and in the fully engorged ticks, respectively. DA-S proportions fluctuated without any distinguishable pattern throughout feeding in a range of 39.8 to 62.5% (Fig. 4b), whereas the proportions of DA-X negatively correlated (r2 = -0.61, P = 0.1) with the trend of free DA, in the range of 12.1 to 46.5% (Fig. 4B).
Quantities of norepinephrine (NE) generally correlated with the quantity of DA with a factor of 10×. A strong correlation was detected between both non-hydrolysed NE and DA (r2 = 0.94, P = 0.0003) and hydrolysed NE and DA (r2 = 0.8, P = 0.01), while weaker and insignificant correlation was observed between sulphatase-treated NE and DA (r2 = 0.48, P = 0.2) (Fig. 5).
Figure 5.
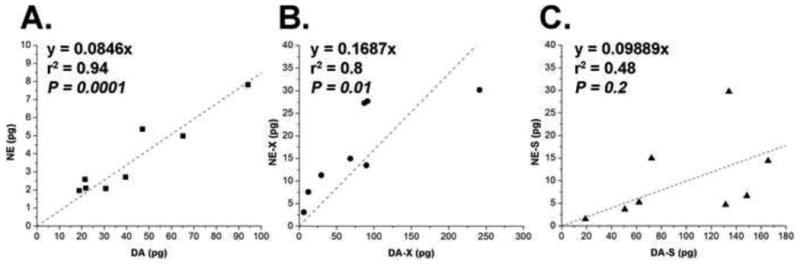
(A) Correlations between the quantities of dopamine (DA) and norepinephrine (NE), (B) between conjugated dopamine (DA-X) and conjugated norepinephrine (NE-X), and (C) between dopamine sulphate (DA-S) and norepinephrine sulphate (NE-S). Plotted values refer to the mean quantities from samples at one-day intervals (pg/tick) of three biological replicates. The linear regression fitting and the significances of the correlation are shown.
3.3. DA in salivary secretions
We measured the quantity of DA and its derivatives in salivary secretions in the D1 and D5. Small amounts of DA in both D1 and D5 female saliva (1.9 pg/μl and 0.6 pg/μl, respectively) were detected. In comparison to the amount of DA extractable from a single SG (21.5 and 94.1 pg/SG in D1 and D5, respectively), the low quantity of DA in the saliva may be the remaining after biodegradation. The majority of the DA derivatives in saliva consisted of DA-S (85.8% and 62.2% of total DA on D1 and D5, respectively) (Fig. 6).
Figure 6.
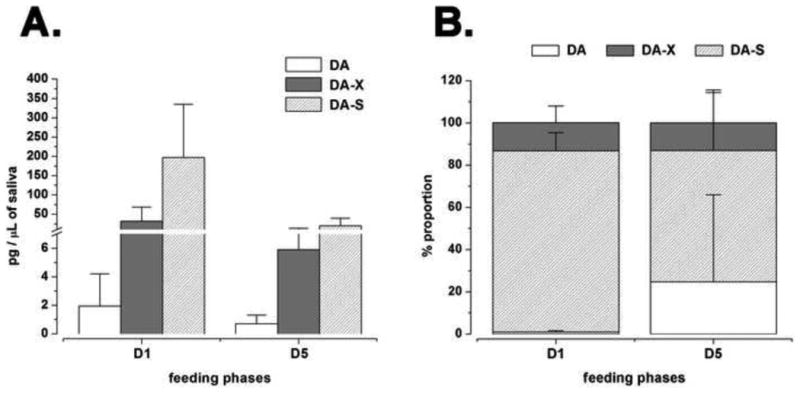
(A) Quantities of dopamine and its conjugates in the tick salivary secretion on day 1 and day 5. Free dopamine (DA), conjugated DA (DA-X), and dopamine sulphate (DA-S) were measured in one day-fed (D1) and five day-fed (D5) female I. scapularis ticks. (B) Proportions of dopamine and its conjugates in tick saliva. The graphs plot the mean values with standard deviation (S.D.) of three biological replicates.
3.4. Effects of pilocarpine on DA levels
We examined the effects of pilocarpine on DA contents in the SG, synganglia, and the haemolymph in D5 ticks. After dorsal applications of pilocarpine (10 mg/ml) or of Hank's medium as a negative control, both groups were incubated for either 0.5 or 4 hours at 37 °C. In this simplified experimental set, we did not measure DA-S separately, so it was included in the hydrolysable DA (DA-X). The amount of DA and DA-X in the SG and synganglia (Fig. 7A,B) were similar to the quantities detected in the tissue-specific measurements (Fig. 2), while the haemolymph displayed very high levels of DA-X, reaching 4 ng/tick (Fig. 7B). There were no significant differences detected in the DA levels and/or proportions between control and PC treatment in any of the three tissues or between 0.5 and 4-hr incubation after PC treatment in any of the three samples (Fig. 7A,B,C).
Figure 7.
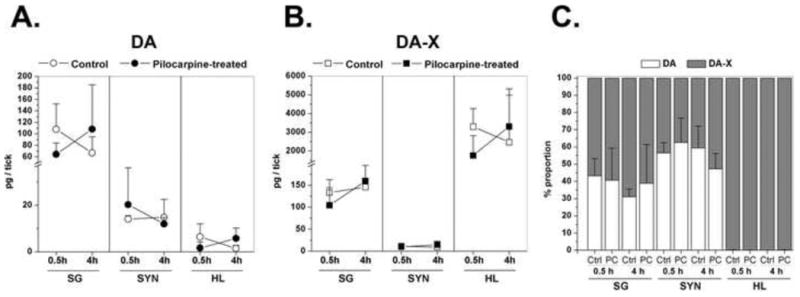
(A) Free dopamine (DA) and (B) dopamine conjugates (DA-X) quantified in salivary glands (SG), synganglia (SYN) and haemolymph (HL) of five day-fed female I. scapularis ticks. Samples are from the ticks that were stimulated by pilocarpine (experimental group) or Hank's buffer solution (control group) for 0.5 hours or 4 hours. (C) Proportions of DA and DA-X. Data were from three biological replicates are presented as means with standard deviation (S.D.). Note that DA-X includes dopamine sulphate (DA-S) in this experimental set.
4. Discussion
Quantification of DA and its conjugated forms using HPLC-ECD provided sufficient sensitivity, accuracy and yield (Fig. 1 in supplementary material). We found large quantities of DA in the SG (up to 100pg/SG, 7.5-fold more than in synganglia) of I. scapularis, supporting the previous study by Kaufman et al. (Kaufman et al., 1999). They showed ∼2 ng/SG in 4-day fed SG of A. hebraeum, a tick species with a much larger body size. Furthermore, the temporal dynamics of DA in the SG indicates that the highest levels of DA on D5 and D6 (Fig. 4A) coincide with the rapid engorgement of blood in our feeding model, during which up to 75% of water from imbibed and digested blood meal is excreted back to a host via SG (Sauer and Hair, 1971; Kaufman and Phillips, 1973). Nevertheless, the role of the SG as the autocrine/paracrine target of DA that triggers tick salivary secretion was also described in our previous studies (Simo et al, 2011; Simo et al., 2013b).
The major portion of DA in the SG is likely from the cytoplasmic pool of the acinar cells, although we do not know the exact cell type responsible for DA production. Luminal secretion of DA will activate DA receptors. We recently described two different salivary DA receptors in I. scapularis: D1 and invertebrate-specific D1-like (InvD1L). The D1 receptor, located on the luminal surface of epithelial cell types, was proposed to function as an activator of inward fluid transport (Simo et al, 2011). The InvD1L receptor, expressed in neuropeptidergic axon terminals and in other luminal axon-like processes (Simo et al, 2013b), was thought to control myoepithelial cells and/or the acinar valve to export the luminal saliva out through the SG ducts. It is also worth noting that the data quantifying DA levels throughout the duration of feeding measured by HPLC-ECD is not congruent with the times of DA-immunoreactive granules in the basal acini, which appeared only 24 to 48 hours after the onset of the feeding (Simo et al, 2011). Therefore, we speculate that DA immunoreactivity likely revealed only a subpopulation of DA or its metabolic variants.
We also detected trace amounts of free DA in the secreted saliva, 1.9 and 0.6 pg/μl (12 and 4 nM) in D1 and D5 samples, respectively (Fig. 6), which was considerably more than the 46 fg/μl previously detected in a much larger Amblyomma tick (Kaufman and Sloley, 1996). It is questionable whether the trace amount of salivary DA could be bioactive in the host after it is further diluted in the host's blood stream.
We expanded our investigation to measure the quantities of conjugated DA that was hydrolysable by sulphatase (presumed DA-3-S and DA-4-S, referred to as DA-S) or by a procedure of boiling under strongly acidic conditions (presumed DA-3-X and DA-4-X, where the X could correspond to glucuronide or other unknown molecules, referred to as DA-X). During the unfed stage, large quantities of DA-X/S were detected in the SG, while the free DA level was very low. Upon initiation of feeding, on D1, the proportions of DA-X and DA-S were significantly reduced and then subsequently increased with the rising quantity of DA until D5. The proportions of DA-X and DA-S were highest on D6 and at FE, respectively (Fig. 4B). On the other hand, in tick synganglia, we found that the free DA form was predominant compared to conjugated DA-X and DA-S (Fig. 2B). Such a ratio was also found in rat brain, where free DA is significantly predominant over DA-S (Buu et al., 1981a). We also detected a large quantity of conjugated DA (∼2 ng/tick) compared to free DA (∼5pg/tick) in the haemolymph of ticks (Fig. 7). The source of the large quantity of DA-X and DA-S in the tick haemolymph could be derived primarily from other non-neuronal tissues, such as the gut. In humans, the mesenteric system is thought to produce about half of the DA and more than 75% of the DA-S in the body (Eisenhofer et al, 1999). The importance of plasma DA-S as a source of DA and NE production via sulphatase-mediated desulphonation in mammals has been debated (Kiyoko et al, 1989; Unger et al, 1980).
DA-S may possess its own bioactivity on the host as a component of saliva. It is difficult to interpret the large amount of DA-S in the saliva (200 pg/μL or 860 nM, 100-fold more than free DA in D1, Fig. 6) as simply inactivated DA due to bioconjugation. Furthermore, it is difficult to determine whether the cytosolic DA sulphotransferases identified in the tick SG (Pichu et al., 2011) are responsible for inactivation of luminal DA in the SG. Earlier reports have shown bioactivity of DA-S, as injection of DA-S in rat brain caused convulsions and seizures independent of the DA receptor (Buu et al., 1981b). Therefore, a role for DA-S as a bioactive salivary component is an open possibility. In this case, some of the DA in the SG may serve as the precursor of DA-S.
A small but significant amount of NE and NE conjugates were detected in the SG extracts, up to 8pg/SG (47fmol) of NE and 60pg/SG (355fmol) of NE-X. Although there is no specific adrenergic receptor described in arthropods, NE has significant levels of cross-activity on DA receptors and on the D1 receptor of I. scapularis in particular (Simo et al, 2013b). The level of NE in salivary secretions was less than quantifiable (lower than ∼100fg). We speculate that constitutive low activity of aromatic amino acid beta hydroxylase (also known as tyramine beta hydroxylase in arthropods) is involved in the conversion of DA to NE. Our data investigating temporal dynamics indicated a generally increasing trend of DA and NE throughout the entire feeding process, although the NE quantity was detected to be ∼5-15-fold less than DA, which is similar to the NE/DA ratio in most invertebrates (Kerkut, 1973). The present data support that NE in the tick SG is a by-product of DA with no clearly specific function.
Finally, we examined the effects of pilocarpine, a cholinomimetic agent that is an agonist of the muscarinic acetylcholine receptor, on DA levels in the SG, the synganglion, and the haemolymph. PC-induced salivary secretion in A. hebraeum begins within 10 min after the injection of PC, and the activity lasts less than one hour (Kaufman, 1978). Because isolated SG do not respond to PC, the cholinergic synapse in the synganglion is thought to be the upstream signal activating salivary secretion (Kaufman et al, 1980). Therefore, the role of DA as a SG autocrine/paracrine factor could be downstream of PC-mediated salivary secretion. We were interested in whether PC-mediated loss (or secretion) of DA is detectable by examining the quantities of DA in each tissue at 0.5 and 4 h after topical application of PC. Although we expected to detect the changes in the DA quantity, such as depletion and replenishment in the organ, which could be the source of the DA involved in salivary secretion, we did not detect any significant changes in the quantity of DA or DA-X between the two different time points or between control and PC treatment. Therefore, the DA involved in the downstream effect of PC on salivary secretion may be a minute quantity compared to the total DA. Alternatively, the DA secreted at the active site may be rapidly recycled in the secretory pool.
In summary, the large quantity of DA and a small but significant quantity of NE were detected in the SG supports the autocrine/paracrine function of DA in the SG of the I. scapularis. In addition, a large quantity of DA-S, but a trace amount of DA in the salivary secretion opens the possibility of DA-S as a bioactive salivary component.
Supplementary Material
Supplementary figure 1. (A) HPLC-Ecd overlaid chromatograms of synthetic catecholamine standards Norepinephrine (NE), Dopamine (DA) and Isoproterenol (ISO). (B) Standard curves of tested catecholamines.
Highlights.
Large quantity of dopamine was detected in the tick salivary glands.
Autocrine/paracrine action of dopamine for salivary secretion is supported.
Tick salivary secretion contains a large amount of dopamine sulphate.
Acknowledgments
This paper is contribution no. 13-xxx-J from the Kansas Agricultural Experiment Station and was supported by National institute of health; Grant Number: R01AI090062
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Juraj Koči, Email: koci@ksu.edu.
Ladislav Šimo, Email: simo@ksu.edu.
References
- Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E. Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity. 2002;16:849–859. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- Anisuzzaman, Islam MK, Alim MA, Miyoshi T, Hatta T, Yamaji K, Matsumoto Y, Fujisaki K, Tsuji N. Longistatin is an unconventional serine protease and induces protective immunity against tick infestation. Molecular and Biochemical Parasitology. 2012;182:45–53. doi: 10.1016/j.molbiopara.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Arakane Y, Lomakin J, Beeman RW, Muthukrishnan S, Gehrke SH, Kanost MR, Kramer KJ. Molecular and functional analyses of amino acid decarboxylases involved in cuticle tanning in Tribolium castaneum. The Journal of Biological Chemistry. 2009;284:16584–16594. doi: 10.1074/jbc.M901629200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman AS, Sauer JR. Tick salivary glands: function, physiology and future. Parasitology. 2004;129 Suppl:S67–81. doi: 10.1017/s0031182004006468. [DOI] [PubMed] [Google Scholar]
- Buu NT, Duhaime J, Savard C, Truong L, Kuchel O. Presence of conjugated catecholamines in rat brain: a new method of analysis of catecholamine sulfates. Journal of Neurochemistry. 1981a;36:769–772. doi: 10.1111/j.1471-4159.1981.tb01655.x. [DOI] [PubMed] [Google Scholar]
- Buu NT, Duhaime J, Kuchel O, Genest J. The convulsive effects of dopamine sulfate conjugates in rat brain. Life Sciences. 1981b;29:2311–2316. doi: 10.1016/0024-3205(81)90564-6. [DOI] [PubMed] [Google Scholar]
- Das S, Banerjee G, DePonte K, Marcantonio N, Kantor FS, Fikrig E. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. The Journal of Infectious Diseases. 2001;184:1056–1064. doi: 10.1086/323351. [DOI] [PubMed] [Google Scholar]
- Decrem Y, Mariller M, Lahaye K, Blasioli V, Beaufays J, Zouaoui Boudjeltia K, Vanhaeverbeek M, Cerutti M, Brossard M, Vanhamme L, Godfroid E. The impact of gene knock-down and vaccination against salivary metalloproteases on blood feeding and egg laying by Ixodes ricinus. International Journal for Parasitology. 2008;38:549–560. doi: 10.1016/j.ijpara.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Coughtrie MW, Goldstein DS. Dopamine sulphate: an enigma resolved. Clinical and Experimental Pharmacology & Physiology. 1999;(26):S41–53. [PubMed] [Google Scholar]
- Francischetti IM, Valenzuela JG, Andersen JF, Mather TN, Ribeiro JM. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: identification of factorfand factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood. 2002;99:3602–3612. doi: 10.1182/blood-2001-12-0237. [DOI] [PubMed] [Google Scholar]
- Gorman MJ, Arakane Y. Tyrosine hydroxylase is required for cuticle sclerotization and pigmentation in Tribolium castaneum. Insect Biochemistry and Molecular Biology. 2010;40:267–273. doi: 10.1016/j.ibmb.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DC, Rosell R, Coons LB, Needham GR. Tick (Acari: Ixodidae) attachment cement and salivary gland cells contain similar immunoreactive polypeptides. Journal of Medical Entomology. 1992;29:305–309. doi: 10.1093/jmedent/29.2.305. [DOI] [PubMed] [Google Scholar]
- Kaufman R, Sloley D. Catabolism of dopamine and 5-hydroxytryptamine by monoamine oxidase in the ixodid tick, Amblyomma hebraeum. Insect Biochemistry and Molecular Biology. 1996;26:101–109. doi: 10.1016/0965-1748(95)00069-0. [DOI] [PubMed] [Google Scholar]
- Kaufman W. The influence of various factors on fluid secretion by in vitro salivary glands of ixodid Ticks. The Journal of Experimental Biology. 1976;64:727–742. doi: 10.1242/jeb.64.3.727. [DOI] [PubMed] [Google Scholar]
- Kaufman WR. The influence of adrenergic agonists and their antagonists on isolated salivary glands of ixodid ticks. European Journal of Pharmacology. 1977;45:61–68. doi: 10.1016/0014-2999(77)90058-9. [DOI] [PubMed] [Google Scholar]
- Kaufman WR. Actions of some transmitters and their antagonists on salivary secretion in a tick. The American Journal of Physiology. 1978;235:R76–81. doi: 10.1152/ajpregu.1978.235.1.R76. [DOI] [PubMed] [Google Scholar]
- Kaufman WR, Aeschlimann AA, Diehl PA. Regulation of body volume by salivation in a tick challenged with fluid loads. The American Journal of Physiology. 1980;238:R102–112. doi: 10.1152/ajpregu.1980.238.1.R102. [DOI] [PubMed] [Google Scholar]
- Kaufman WR, Flynn PC, Reynolds SE. Cuticular plasticization in the tick, Amblyomma hebraeum (Acari: Ixodidae): possible roles of monoamines and cuticular pH. The Journal of Experimental Biology. 2010;213:2820–2831. doi: 10.1242/jeb.044412. [DOI] [PubMed] [Google Scholar]
- Kaufman WR, Harris RA. Neural pathways mediating salivary fluid secretion in the ixodid tick Amblyomma hebraeum. Canadian Journal of Zoology. 1983;61:1976–1980. doi: 10.1139/z80-148. [DOI] [PubMed] [Google Scholar]
- Kaufman WR, Phillips JE. Ion and Water Balance in the Ixodid Tick Dermacentor Andersoni: I. Routes of Ion and Water Excretion. Journal of Experimental Biology. 1973;58:523–536. [Google Scholar]
- Kaufman WRS, D B, Tatchell RJ, Zbitnew GL, Diefenbach TJ, Goldberg JI. Quantification and cellular localization of dopamine in the salivary gland of the ixodid tick Amblyomma hebraeum. Experimental & Applied Acarology. 1999;23:251–265. [Google Scholar]
- Kerkut GA. Catecholamines in invertebrates. British Medical Bulletin. 1973;29:100–103. doi: 10.1093/oxfordjournals.bmb.a070976. [DOI] [PubMed] [Google Scholar]
- Kiyoko H, Atsushi Y, Takanobu Y, Hiroshi W, Toshio O. Contents of dopamine sulfoconjugate isomers and their desulfation in dog arteries. Biochemical Pharmacology. 1989;38:1891–1895. doi: 10.1016/0006-2952(89)90486-3. [DOI] [PubMed] [Google Scholar]
- Koci J, Simo L, Park Y. Validation of internal reference genes for real-time quantitative polymerase chain reaction studies in the tick, Ixodes scapularis (Acari: Ixodidae) Journal of Medical Entomology. 2013;50:79–84. doi: 10.1603/me12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwain JL, Essenberg RC, Sauer JR. Oral secretion elicited by effectors of signal transduction pathways in the salivary glands of Amblyomma americanum (Acari: Ixodidae) Journal of Medical Entomology. 1992;29:41–48. doi: 10.1093/jmedent/29.1.41. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Sugimoto C, Sako Y, Ohashi K, Musoke A, Shubash M, Onuma M. Molecular characterization of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infection and Immunity. 1999;67:1652–1658. doi: 10.1128/iai.67.4.1652-1658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Deponte K, Marcantonio N, Liang X, Royce TE, Nelson KF, Booth CJ, Koski B, Anderson JF, Kantor F, Fikrig E. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PloS One. 2007;2:e451. doi: 10.1371/journal.pone.0000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichu S, Yalcin EB, Ribeiro JM, King RS, Mather TN. Molecular characterization of novel sulfotransferases from the tick, Ixodes scapularis. BMC Biochemistry. 2011;12:32. doi: 10.1186/1471-2091-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot PP, Couvreur B, Denis V, Brossard M, Vanhamme L, Godfroid E. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine. 2007;25:3284–3292. doi: 10.1016/j.vaccine.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Makoul GT, Levine J, Robinson DR, Spielman A. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. The Journal of Experimental Medicine. 1985;161:332–344. doi: 10.1084/jem.161.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JR, Hair JA. Water balance in the lone star Tick (Acarina: Ixodidae): the effects of relative humidity and temperature on weight changes and total water content. Journal of Medical Entomology. 1971;8:479–485. doi: 10.1093/jmedent/8.5.479. [DOI] [PubMed] [Google Scholar]
- Schmidt SP, Essenberg RC, Sauer JR. A dopamine sensitive adenylate cyclase in the salivary glands of Amblyomma americanum (L.) Comparative Biochemistry and Physiology. C: Comparative Pharmacology. 1982;72:9–14. doi: 10.1016/0306-4492(82)90197-6. [DOI] [PubMed] [Google Scholar]
- Simo L, Koci J, Park Y. Receptors for the neuropeptides, myoinhibitory peptide and SIFamide, in control of the salivary glands of the blacklegged tick Ixodes scapularis. Insect Biochemistry and Molecular Biology. 2013a;43:376–387. doi: 10.1016/j.ibmb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo L, Koci J, Kim D, Park Y. Invertebrate specific D1-like dopamine receptor in control of salivary glands in the black-legged tick Ixodes scapularis. The Journal of Comparative Neurology. 2013b doi: 10.1002/cne.23515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo L, Koci J, Zitnan D, Park Y. Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PloS One. 2011;6:e16158. doi: 10.1371/journal.pone.0016158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo L, Slovak M, Park Y, Zitnan D. Identification of a complex peptidergic neuroendocrine network in the hard tick, Rhipicephalus appendiculatus. Cell and Tissue Research. 2009a;335:639–655. doi: 10.1007/s00441-008-0731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo L, Zitnan D, Park Y. Two novel neuropeptides in innervation of the salivary glands of the black-legged tick, Ixodes scapularis: myoinhibitory peptide and SIFamide. The Journal of Comparative Neurology. 2009b;517:551–563. doi: 10.1002/cne.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo L, Zitnan D, Park Y. Neural control of salivary glands in ixodid ticks. Journal of Insect Physiology. 2012;58:459–466. doi: 10.1016/j.jinsphys.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimnell AR, Davies GM, Lissina O, Hails RS, Nuttall PA. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine. 2005;23:4329–4341. doi: 10.1016/j.vaccine.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Unger T, Buu NT, Kuchel O, Schürch W. Conjugated dopamine: peripheral origin, distribution, and response to acute stress in the dog. Canadian Journal of Physiology and Pharmacology. 1980;58:22–27. doi: 10.1139/y80-005. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Charlab R, Mather TN, Ribeiro JM. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. The Journal of Biological Chemistry. 2000;275:18717–18723. doi: 10.1074/jbc.M001486200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Bruno JF, Luft BJ. Identification of novel tick salivary gland proteins for vaccine development. Biochemical and Biophysical Research Communications. 2005;326:901–904. doi: 10.1016/j.bbrc.2004.11.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. (A) HPLC-Ecd overlaid chromatograms of synthetic catecholamine standards Norepinephrine (NE), Dopamine (DA) and Isoproterenol (ISO). (B) Standard curves of tested catecholamines.


