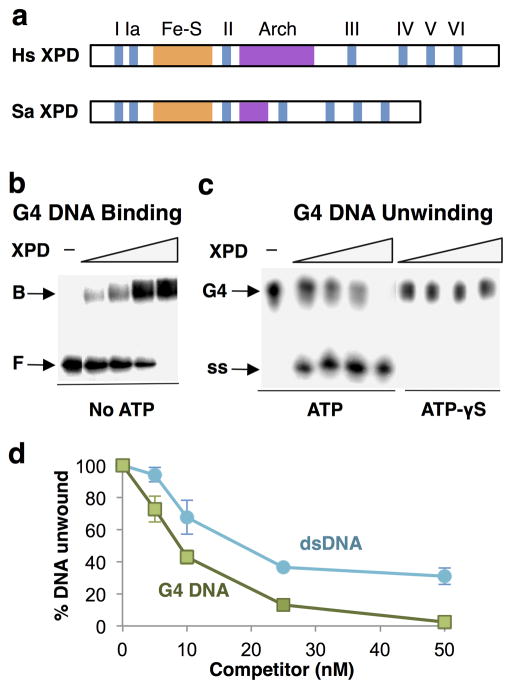
Figure 1. XPD is a robust G4 DNA helicase.
(a) Domain structure of human (Hs) and S. acidocaldarius (Sa) XPD, showing conserved helicase motifs I–VI and Fe-S and Arch domains. (b) Gel mobility shift assay of binding by XPD (0, 25, 50, 100 and 200 nM) to 32P-G4 DNA. Arrows indicate bound (B) and free (F) DNA. (c) Assay of unwinding of 32P-G4 DNA by XPD (0, 25, 50, 100 and 200 nM) in the presence of 1 mM ATP (left) or 1 mM ATP-γS (right). Arrows indicate G4 DNA substrate and single-stranded (ss) products of unwinding. (d) Competition of 32P-G4 DNA unwinding by indicated amount of unlabeled G4 DNA or dsDNA.