Abstract
Bees visit flowers to collect nectar and pollen that contain nutrients and simultaneously facilitate plant sexual reproduction. Paradoxically, nectar produced to attract pollinators often contains deterrent or toxic plant compounds associated with herbivore defence. The functional significance of these nectar toxins is not fully understood, but they may have a negative impact on pollinator behaviour and health, and, ultimately, plant pollination. This study investigates whether a generalist bumblebee, Bombus terrestris, can detect naturally occurring concentrations of nectar toxins. Using paired-choice experiments, we identified deterrence thresholds for five compounds found in the nectar of bee-pollinated plants: quinine, caffeine, nicotine, amygdalin and grayanotoxin. The deterrence threshold was determined when bumblebees significantly preferred a sucrose solution over a sucrose solution containing the compound. Bumblebees had the lowest deterrence threshold for the alkaloid quinine (0.01 mmol l−1); all other compounds had higher deterrence thresholds, above the natural concentration range in floral nectar. Our data, combined with previous work using honeybees, suggest that generalist bee species have poor acuity for the detection of nectar toxins. The fact that bees do not avoid nectar-relevant concentrations of these compounds likely indicates that it is difficult for them to learn to associate floral traits with the presence of toxins, thus maintaining this trait in plant populations.
KEY WORDS: Pollinator, Bombus terrestris, Nectar toxin, Grayanotoxin, Behaviour, Deterrence threshold
INTRODUCTION
Pollination is a key ecosystem service provided by flower-visiting animals. It is estimated that over 87% of the world's angiosperm species are animal pollinated and thus potentially influenced by pollinator foraging behaviour (Ollerton et al., 2011) because patterns of floral visitation by nectar- and pollen-collecting animals influence the quantity and quality of pollination events (Aizen and Harder, 2007). In order to attract vital pollinators, many plants produce sugar-rich nectar, the primary function of which is to reward animals for visiting flowers (Heil, 2011). Nectar is the principal source of carbohydrates for most flower-visiting insects (Michener, 1974; Nicolson, 2011); however, this reward can paradoxically contain low concentrations of potentially deterrent or toxic plant compounds. These secondary compounds, such as alkaloids, phenolics and non-protein amino acids, are produced in plant tissues as a means of chemical defence against herbivores (Adler, 2000; Baker and Baker, 1975; Baker, 1977). Expression of toxins in nectar can be affected by herbivorous attack, and so the naturally occurring concentrations to which pollinators are exposed can fluctuate (Adler et al., 2006). Many adaptive functions have been proposed to explain the presence of these compounds in nectar, including deterring nectar robbers (Baker et al., 1978; Janzen, 1977), altering pollinator behaviour (Baker and Baker, 1975; Ehlers and Olesen, 1997; Rhoades and Bergdahl, 1981; Wright et al., 2013) and providing antimicrobial properties that can benefit both the plant [by preserving the nectar quality for pollinators (Hagler and Buchmann, 1993; Adler, 2000)] and the pollinators [by medicating against harmful pathogens and parasites (Manson et al., 2010)]. The functional significance of toxins in nectar is likely to depend on the ecological context and the nature of the toxin, but we still know relatively little about their influence on pollinators.
Understanding the significance that nectar toxins have on plant–pollinator interactions requires knowledge of how pollinators alter their behaviour in response to consumption of these compounds. For example, pollinators may avoid toxin-contaminated nectar: honeybees reject nectar containing nicotine, and several wild bee species avoid foraging on plants containing high concentrations of the alkaloid gelsemine (Adler and Irwin, 2005; Detzel and Wink, 1993; Hagler and Buchmann, 1993). Occasionally, the opposite has been demonstrated: for example, free-flying honeybees prefer solutions containing low concentrations of the alkaloid caffeine, and were even found to increase visitation rates (Hagler and Buchmann, 1993) or learn floral traits faster when it was present (Wright et al., 2013). However, most plant secondary compounds are toxic to animals (Rosenthal and Berenbaum, 1992), and their ingestion could represent a significant form of physiological stress that would require energy or resources to metabolise or cope with the toxin (Després et al., 2007; Schuler, 2011). If consuming such plant compounds is costly, one would predict that when nectar-feeders can detect toxins, they should learn to avoid plant species offering toxic nectar (Adler and Irwin, 2005; Detzel and Wink, 1993; Glendinning, 2002; Hagler and Buchmann, 1993). It remains unclear, however, whether most pollinators can detect or are deterred by naturally occurring concentrations of secondary compounds in nectar. If these compounds do not deter pollinators, any benefit to the plant of their presence [e.g. the deterrence of nectar robbers (Janzen, 1977) or suppression of nectar quality-altering microbes (Adler, 2000)] would allow the trait to be maintained in the plant population.
Bumblebees such as the widespread species Bombus terrestris are ecologically and economically important pollinators. They are generalists that visit many plant species, including those containing nectar toxins (Detzel and Wink, 1993; Kretschmar and Baumann, 1999; London-Shafir et al., 2003; Stout et al., 2006). Several studies have shown that when bumblebees and honeybees detect toxins such as the bitter-tasting alkaloid quinine, they will learn to avoid floral
List of abbreviations
- GLM
generalised linear model
- Grs
gustatory receptors
- GTX
grayanotoxin
- LC–MS
liquid chromatography–mass spectrometry
- LSD
least significant difference
- NMR
nuclear magnetic resonance
traits associated with the compound's presence in sucrose rewards (Chittka et al., 2003; Mustard et al., 2012; Wright et al., 2010). However, many of these studies use concentrations of toxins several orders of magnitude beyond their concentration in nectar. Whether bumblebees can detect the same compounds at concentrations encountered in floral nectar remains unknown.
Here, we performed a series of experiments to test whether B. terrestris was deterred by naturally occurring concentrations of nectar toxins in sucrose solutions. This study is the first to determine the deterrence thresholds of nectar toxins for a Bombus species. We discuss the resultant implications concerning bee gustatory acuity and bee health, as well as how our results add to the growing body of literature concerning the functional significance of toxins in nectar.
RESULTS
Bumblebees are not deterred by naturally occurring concentrations of nectar toxins
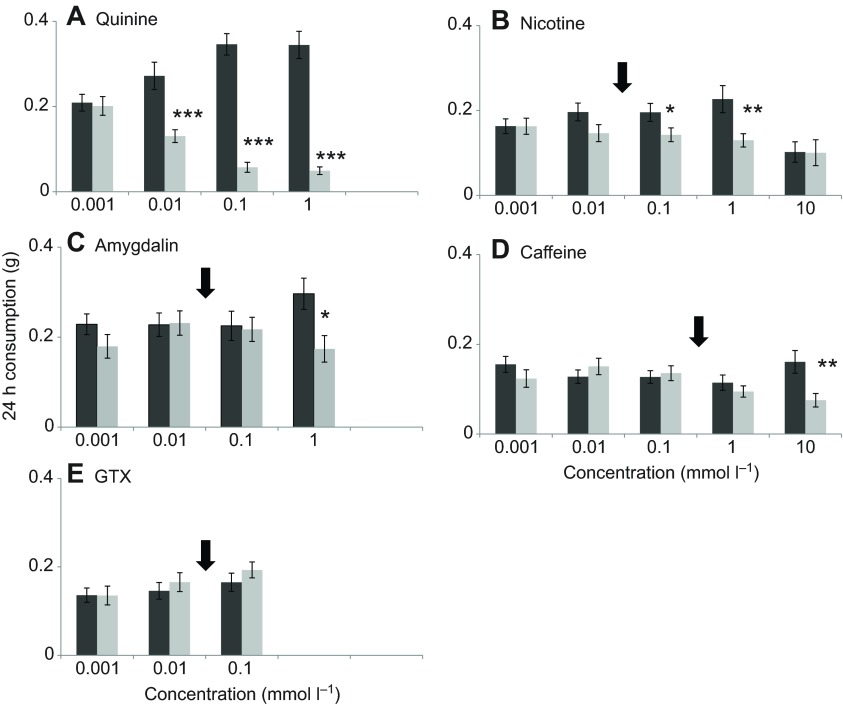
Bumblebees failed to be deterred by any of the compounds tested [nicotine, amygdalin, caffeine and grayanotoxin (GTX)] at naturally occurring concentrations in nectar (Fig. 1). In contrast, the alkaloid quinine was readily avoided even at doses as low as 0.01 mmol l−1 (GLM, χ32=59.2, P<0.001; Fig. 1A). The pairwise comparison illustrated that bumblebees preferred the pure sucrose solution (the internal control) over a quinine concentration of 0.01 mmol l−1 (P<0.001), and continued to exhibit this preference for the two highest quinine concentrations (Fig. 1A).
Fig. 1.
Mean (±s.e.m.) consumption (g), controlled for evaporation by Bombus terrestris of 0.5 mol l−1 sucrose solution, with (light grey bars) or without (dark grey bars) one of five nectar toxins. Where bars are missing, assays were not completed because of limited availability of compounds. Asterisks indicate significant differences between consumption of two solutions at a given concentration according to least significant difference (LSD) post hoc comparisons (*P<0.05; **P<0.01; ***P<0.001). Black arrows represent naturally occurring concentrations of the compound in floral nectar.
By contrast, bumblebees had higher deterrence thresholds for the other alkaloids. While nicotine was deterrent at 0.1 mmol l−1 (GLM, χ32=20.2, P<0.001; Fig. 1B), in tobacco flower nectar it has been found at concentrations of 0.015 mmol l−1 (Tadmor-Melamed et al., 2004), nearly seven times lower than the deterrence threshold of B. terrestris. The preference of the bumblebees for the pure sucrose solution continued for the 1 mmol l−1 nicotine concentration, but surprisingly, individuals fed the highest concentration of nicotine, 10 mmol l−1, did not show a preference for either solution (P=0.974). They did, however, consume less total food than individuals fed any of the four lower concentrations (F=3.44, P=0.010; Fig. 1B). The deterrence threshold for another nectar alkaloid, caffeine, was 10 mmol l−1 and was the highest of all the compounds we tested (GLM, χ32=10.0, P<0.01; Fig. 1D). This value is 20 times higher than the highest caffeine concentration found in floral nectar, 0.5 mmol l−1 (Kretschmar and Baumann, 1999), and three orders of magnitude higher than the deterrence threshold for the alkaloid quinine.
The bumblebees' deterrence threshold for the cyanogenic glycoside amygdalin was 1 mmol l−1 (GLM, χ32=3.8, P<0.05; Fig. 1C) – more than 60 times greater than the highest concentration of amygdalin found in floral nectar (0.015 mmol l−1) (London-Shafir et al., 2003). Finally, bumblebees could not detect GTX in any of the concentrations we tested (GLM, χ32=0.604, P=0.739; Fig. 1E).
Compensative feeding does not occur for all nectar toxins
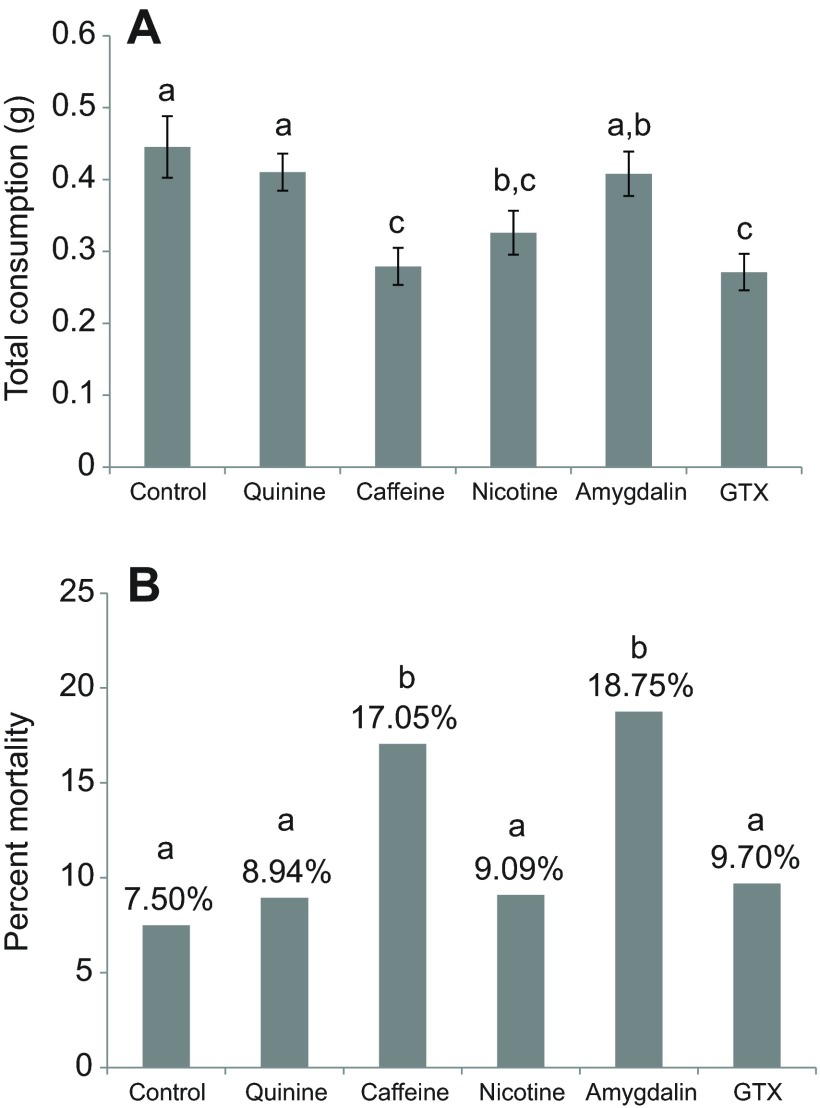
The total amount of food consumed (sucrose solution + sucrose solution containing toxic compounds) by bumblebees differed significantly depending upon which toxin was consumed (GLM, χ32=70.3, P<0.001; Fig. 2A). The total consumption of individuals fed solutions containing caffeine, nicotine and GTX was significantly lower than that of the control bumblebees (P<0.001, P=0.002 and P<0.001, respectively). By contrast, the total consumption of bumblebees fed quinine and amygdalin did not differ from control bumblebees (P=0.244 and P=0.803, respectively). The analysis of total food consumption was undertaken for the lowest concentration of toxin tested, 0.001 mmol l−1, because bumblebees could not detect any of the toxins at this level. However, the same pattern was found when all concentrations for which the design was fully factorial across all toxins were analysed (0.001, 0.01 and 0.1 mmol l−1): bumblebees fed caffeine, nicotine and GTX consumed significantly less total food than controls (GLM, χ32=30.3, P<0.001).
Fig. 2.
Mean (±s.e.m.) (A) total consumption (g), controlled for evaporation of solutions at lowest concentration (0.001 mmol l−1) for each nectar toxin, and (B) mortality of Bombus terrestris fed five different nectar toxins. Control bumblebees were fed 0.5 mol l−1 sucrose in both solutions and so had no exposure to any toxin. N=40 bees per toxin per concentration. Different lowercase letters represent significant (P<0.05) differences in total consumption between compounds according to LSD post hoc comparison.
The toxins also had a significant effect on bumblebee mortality (GLM, χ32=15.9, P=0.007). Bumblebees fed amygdalin and caffeine had significantly higher mortality rates than individuals fed any of the other compounds or control bumblebees (P=0.027 and P=0.045, respectively; Fig. 2B). Survival of the bees fed GTX, nicotine or quinine did not differ from that of the control bumblebees.
DISCUSSION
Our experiments show that bumblebees are not deterred by a variety of naturally occurring levels of nectar toxins. This finding has important implications for bumblebee health and for plant–pollinator interactions among Bombus-pollinated plants that produce toxins in their nectar, such as rhododendron (containing GTX) (Stout et al., 2006) and almond tree species (containing amygdalin) (Thomson and Goodell, 2001). Because the compounds we tested did not have repellent effects on bumblebees at nectar-relevant concentrations, these pollinators are unlikely to alter their behaviour to avoid flowers with such compounds.
Bees have poor acuity for toxins in nectar
Our data, combined with those from previous studies using honeybees, demonstrate that generalist bees have relatively low sensitivity for plant toxins in sucrose solutions. Previous work has determined honeybee deterrence thresholds for caffeine, quinine and amygdalin. This work has consistently found that honeybees do not respond to levels of these compounds less than 10 mmol l−1 (Mustard et al., 2012; Wright et al., 2010). For caffeine, the deterrence threshold concentrations for honeybees and bumblebees are similar; however, bumblebees were more sensitive to amygdalin and quinine in our assays (deterrence thresholds of 1 and 0.01 mmol l−1, respectively). Other insect taxa have greater gustatory acuity for these compounds; fruit flies, for example, have deterrence thresholds for caffeine and quinine that are 10–100 times lower than those of bees (Sellier et al., 2011). Similarly, gypsy moth larvae (Lymantria dispar) are deterred by caffeine at levels 100 times lower than bees (Shields et al., 2008).
Generalist bee species may have poor acuity for the detection of toxins in nectar because they have few gustatory receptors (Grs) that can detect these compounds. For example, the honeybee genome encodes only 10 orthologous genes for g-protein coupled Grs (Robertson and Wanner, 2006). This is in contrast to dipteran species such as fruit flies and the mosquito Anopheles gambiae, which have many more genes for Grs (flies: 68; A. gambiae: 76) (Dunipace et al., 2001; Hill et al., 2002; Robertson et al., 2003; Scott et al., 2001). The greater relative diversity of Grs in flies and other insects probably reflects stronger selection for the detection of toxins in food in these species (Robertson and Wanner, 2006).
It is possible that natural selection for the ability to detect plant toxins has not been strong enough to force diversification of eusocial bees' Grs to improve gustatory acuity for these chemicals. This may be a consequence of eusociality, where individual bees are the consumers, but selection pressures act on the colony as the reproductive unit. In solitary animals, the individual bears the fitness cost of toxin consumption. In eusocial honeybees and bumblebees, foragers collect food for the entire colony. If a forager ate nectar contaminated with toxins that it could not detect, it might die, but with little impact on the fitness of the colony [though more impact on bumblebees as compared with honeybees, because of their relatively small colonies (Khoury et al., 2011)]. Selection for the ability to detect toxins would only occur when the queen, and therefore the fitness of the colony, was affected by toxins in nectar.
Our results indicate that out of the classes of toxic compounds tested, individuals of the species B. terrestris are relatively good at detecting and avoiding alkaloids. However, even within this specific class of compounds, the deterrence thresholds varied across four orders of magnitude for different chemicals (i.e. caffeine, nicotine and quinine). Alkaloids are one of the most common and chemically diverse groups of plant compounds, with more than 12,000 structures described (Wink, 1993). The common frequency with which alkaloids are found in higher plants and their toxicity has led insects to develop the ability to detect and reject these chemicals in their food. The diverse chemical structures within alkaloids, however, makes some easier to detect than others.
Total consumption of solutions is affected by toxins in nectar
Our results indicate that when bumblebees consume low, nectar-relevant doses of caffeine, nicotine and GTX, their total intake of food was depressed, regardless of whether they could readily distinguish the two solutions. A study on D. melanogaster found the same phenomenon: flies ate less total sucrose solution when the alkaloids lobeline, nicotine and strychnine were present (Sellier et al., 2011). This reduction in intake of all solutions after toxin consumption may be due to post-ingestive detection of the toxins, which is modulating appetite (Wright et al., 2010). In addition, in our study, bumblebees fed the 10 mmol l−1 nicotine solution consumed equal, but very small, amounts of both solutions, even though their deterrence threshold was at a lower concentration (0.1 mmol l−1; Fig. 1B). Consumption of this concentration of nicotine could have damaged chemosensory sensilla or gustatory receptor neurons of individuals, preventing them from detecting nicotine even though they were capable of doing so at lower concentrations (0.1 mmol l−1) (Sellier et al., 2011).
Bumblebee colonies must reach a minimum size in order to produce new queens and males (Müller and Schmid-Hempel, 1992). If consumption of toxins in floral nectar causes appetite suppression in foraging workers, colonies may not reach this threshold size as early in the season or at all. This could result in a decrease in queen and male production, and, because bumblebees have an annual life cycle, could have a substantial population-level effect (Gill et al., 2012; Henry et al., 2012; Whitehorn et al., 2012).
Functional significance of nectar toxins
Bumblebees are generalist pollinators, and based on the large percentage of plants that have toxins in their nectar (Baker and Baker, 1975; Baker et al., 1978), it is likely that bumblebees encounter these kinds of toxins often (Adler and Irwin, 2005; Stephenson, 1982; Stout et al., 2006). It is possible that legitimate pollinators such as bumblebees have therefore selected for concentrations of toxins in floral nectar that remain below their deterrence level (Wright et al., 2013). For example, if a honeybee learns to associate floral traits with bad-tasting nectar, it will avoid flowers with these traits (Wright et al., 2010) and will potentially communicate the poor quality of the nectar to other colony members or not recruit them to this food source (Tan et al., 2012). In this way, individual bees could drive natural selection towards concentrations of these compounds in nectar that are below their deterrence threshold (Wright et al., 2013; Wright et al., 2010).
Our data suggest that in the field, low levels of toxic compounds in nectar do not affect bumblebee foraging behaviour. These findings are in contrast to those of a similar study investigating the gustatory responses of bumblebees in response to different sugars, where nectar relevant concentrations and sugar identity were shown to impact bumblebee preference (Mommaerts et al., 2013). Bumblebee-pollinated plants containing toxic compounds in their nectar would not suffer from reduced pollination, thus allowing this plant trait to be maintained if it conferred any fitness benefit to the plant. Selection for the production of toxins in nectar is likely to be the result of other factors affecting nectar secretion and production, such as nectar robbery, damage from herbivores, or reduction of nectar quality due to microorganisms. For example, nectar toxins could be toxic or deterrent to nectar thieves but not deter legitimate pollinators; thus they act in a similarly selective manner to morphological characters such as sticky peduncles or narrow corolla tubes (Janzen, 1977; Stephenson, 1982).
This is the first assay to report that the deterrence thresholds of bumblebees are well above nectar-relevant concentrations of toxic compounds in Bombus-pollinated plants. Our data are also the first to provide concentrations that inhibit feeding of the bumblebee for some chemicals commonly found in floral nectar, and to indicate that the acuity of this generalist bumblebee for nectar toxins is poor in comparison to other insect species. This work adds to the growing body of research on the functional significance of nectar toxins on plant–pollinator interactions and the impacts of these chemicals on bee health.
MATERIALS AND METHODS
Subjects
Bombus terrestris dalmatinus (Linnaeus 1758) workers from four colonies (Agralan Ltd, Swindon) were used for each secondary compound assay (total 12 colonies). Prior to use, colonies were maintained at 25–30°C in 24 h darkness and fed commercial pollen and Biogluc (Agralan) bee food ad libitum.
Secondary compounds
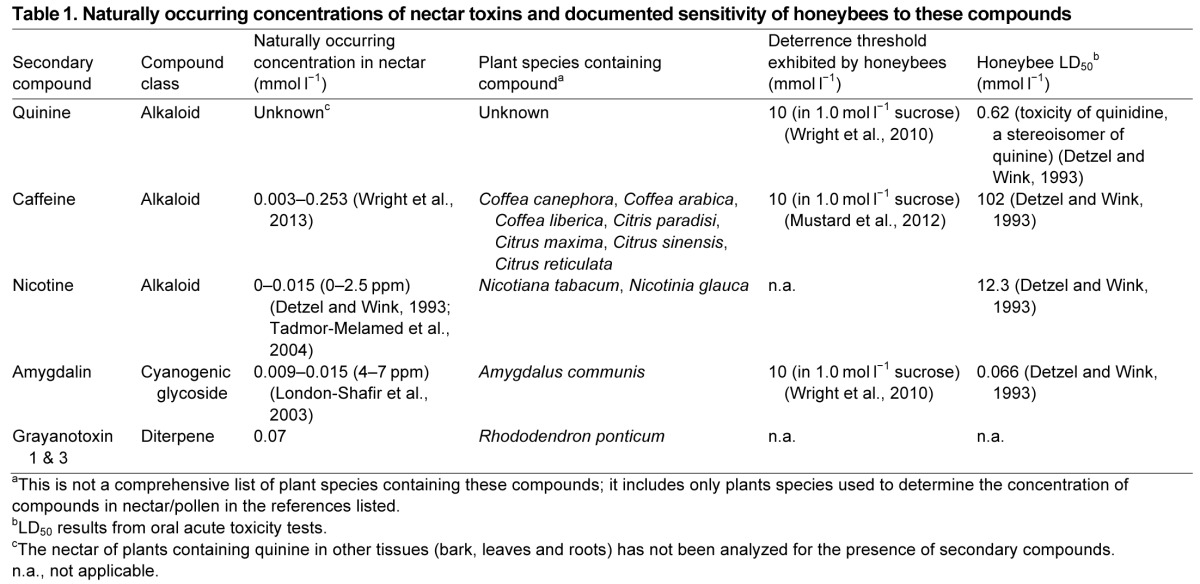
Five compounds were investigated: quinine, caffeine, nicotine, amygdalin and grayanotoxins (GTX) (see Table 1). With the exception of the compound quinine, and to a large extent nicotine, these compounds are known to naturally occur in floral nectar of plant species foraged on by bees (London-Shafir et al., 2003; Raguso et al., 2003; Roubik, 2002; Singaravelan et al., 2006; Stout et al., 2006; Tadmor-Melamed et al., 2004; Thomson and Goodell, 2001). All of the compounds except for GTX 1 were supplied by Sigma-Aldrich (Dorset, UK). GTX (a mixture of GTX 1 and 3) was isolated from flowers of Rhododendron ponticum from the UK using prep-HPLC. Flowers of R. ponticum were harvested from the Isle of Cumrae, Millport, Scotland, and air dried. Dried flowers (100 g) were extracted into 1 l methanol at room temperature for 24 h. The extract was evaporated to dryness and redissolved in 500 ml water and partitioned with hexane (500 ml) twice. The water fraction was further partitioned with 300 ml chloroform four times and the chloroform partition was evaporated under reduced pressure to dryness, redissolved in 10 ml methanol and filtered through a 0.45 μm acrodisc. A 10 μl sample was diluted into 990 μl methanol and a 10 μl aliquot of this diluted sample was injected directly onto the liquid chromatography–mass spectrometry (LC–MS) system. LC–MS analysis was carried out using a Waters Alliance LC solvent delivery system with a ZQ MS detector on a Phenomenex Luna C18(2) column (150×4.0 mm i.d., 5 μm particle size) operating under gradient conditions, with A=MeOH, B=H2O, C=1% HCO2H in MeCN; A=0%, B=90% at t=0 min; A=90%, B=0% at t=20 min; A=90%, B=0% at t=30 min; A=0%, B=90% at t=31 min; column temperature 30°C and flow rate of 0.5 ml min−1. GTX 3 was purchased commercially (Sigma-Aldrich, Dorset, UK) and used as a chromatographic standard to generate a calibration curve for this compound by quantification of the [M-H+formate]− molecular ion in negative mode with m/z=415.3 and eluting at 6.71 min. A second, more abundant [M-H]− ion with m/z=411.1 corresponded to the molecular weight of GTX 1 and eluted at 8.1 min. Using this method, the two GTXs were separated by over 1 min so they could be purified from the fraction by HPLC by collecting fractions by time. HPLC was carried out using a semi-preparative Phenomenex Luna C18(2) column (150×10.0 mm i.d., 5 μm particle size) operating under the same elution programme as described above but with an increased flow of 5 ml min−1 on a Waters Alliance LC system and a Waters fraction collector. Aliquots of 100 μl were injected directly onto the column, the eluent was collected in 30 s batches and each collection was analysed directly by LC-MS as described above to determine the content. GTXs are diterpenoids with no chromophore, so they cannot be detected by their UV absorbance. Isolation of 4 ml of the methanol-soluble partition yielded 20 mg of the main compound (GTX 1) and 1 mg GTX 3 identified earlier by comparison with an authentic standard. The major compound was evaporated to dryness and subjected to nuclear magnetic resonance (NMR) spectroscopy. NMR spectra were acquired in MeOH-d4 at 30°C on a Bruker Avance 400 MHz instrument. Standard pulse sequences and parameters were used to obtain 1D 1H and 1D 13C spectra. Chemical shift referencing was carried out with respect to internal tetramethylsilane at 0.00 ppm and verified as GTX 1 by comparison with published data (Burke and Doskotch, 1990).
Table 1.
Naturally occurring concentrations of nectar toxins and documented sensitivity of honeybees to these compounds
Nectar was collected from R. ponticum on the Isle of Cumrae. A 20 μl aliquot was diluted to 200 μl and injected directly on the LC–MS as described above, and the concentration of compounds present in samples from nectar were quantified in this nectar sample against calibration curves of authentic samples for both GTX 1 isolated here and commercial GTX 3.
Quinine has not been reported in floral nectar, but it is widely used in behavioural studies of honeybees and bumblebees as an aversive stimulus (Chittka et al., 2003; Mustard et al., 2012), and is known to be repellent. We used it as a positive control. The concentrations at which the remaining secondary compounds occur in floral nectar have been previously determined (see Table 1), except for GTX, whose nectar concentration was determined in this study.
Experimental protocol
We determined the deterrence threshold for each secondary compound using a paired-choice assay in which bumblebees were offered two sucrose solutions, one with and one without the compound at a variety of concentrations. Sucrose solutions [0.5 mol l−1, within the range found in the nectar of bee-pollinated flowers (Baker, 1975)] were made by mixing grade II sucrose (Sigma-Aldrich) with deionised water. Serial dilutions were performed to obtain different concentrations of each secondary compound [range of 0.001–10 mmol l−1, encompassing the naturally occurring concentrations of the compounds in floral nectar (Detzel and Wink, 1993; Kretschmar and Baumann, 1999; London-Shafir et al., 2003; Tadmor-Melamed et al., 2004; Wright et al., 2013)], depending on the toxicity and availability of each compound.
Worker bumblebees from each colony were removed and placed into individual plastic containers. Nest bumblebees (spending most of their time caring for brood inside the nest, never foraging) were avoided by refraining from using the smallest workers (Goulson et al., 2002). Bees were chilled on ice for ~3 min or until movement slowed, measured (body length, thorax and abdomen width) and weighed, and randomly allocated to a toxin concentration. Each bee remained in a separate container and was allowed to acclimate for at least 1 h. Forty bumblebees, 10 from each of four colonies, were allocated to each of the concentrations of each compound.
Assays were conducted in 650 ml plastic containers (160×110×45 mm) with lids containing 1 mm diameter ventilation holes. The containers had three additional 10 mm diameter holes on three of the four sides, where feeding tubes could be inserted horizontally. Feeding tubes were 3 ml centrifuge tubes with four 2 mm holes: bees could alight on the tubes and feed from the openings. Bees were given a choice between two solutions: a 0.5 mol l−1 sucrose solution (internal control), and an identical 0.5 mol l−1 sucrose solution containing the toxin. Bees were also supplied with a third tube containing deionised water. Tubes were weighed prior to being inserted into the container and the bee was left to feed for 24 h in growth cabinets at 28°C, 60% relative humidity and 24 h darkness, mimicking nest conditions (Heinrich, 2004). Feeding tubes were then reweighed and the amount of food consumed from each was calculated. Identical setups containing no bees were used daily to control for the change in tube weight due to evaporation (external controls) and the consumption per bee (g) was adjusted accordingly. At least eight of these control setups were run for each concentration of each compound. Data from individual bumblebees were only used in the analysis if bees were still alive at the end of the 24 h test period.
Forty control bumblebees were fed 0.5 mol l−1 sucrose in both tubes (10 from each of four colonies) for comparison with bees fed toxins.
Data analysis
Consumption data for each of the six compounds were analysed using generalised linear models (GLMs) with repeated measures. Concentration and solution type (presence/absence of the toxin) were included in the model as main effects and a significant interaction between the two indicated the presence of a deterrence threshold for a given compound. A least significant difference (LSD) post hoc comparison was used for all pairwise comparisons. Total consumption (cumulative consumption by each bumblebee, both the internal control and the solution containing the toxin) was compared between secondary compounds using concentrations for which the design was fully factorial (the three lowest concentrations tested: 0.001, 0.01 and 0.1 mmol l−1) using GLMs. Logistic regression was utilized to determine whether there was a significant effect of toxin on mortality. All analyses were carried out using the statistical package SPSS Statistics, version 20 (IBM).
ACKNOWLEDGEMENTS
We gratefully acknowledge P. Egan for chemical analysis of Rhododendron nectar as well as for his valuable advice. We thank D. Stabler for his input into bumblebee feeding techniques and setup. We also thank the Trinity College Dublin School of Natural Sciences technicians, P. Stafford, A. Boyce, M. Linnie, S. McNamee, P. Coughlan and J. Stone. We thank Dr Nigel C. Veitch at Royal Botanic Gardens Kew for providing NMR data and verifying the ID of grayanotoxin.
Footnotes
Competing interests
The authors declare no competing financial interests.
Funding
This project was funded by Science Foundation Ireland [grant number 10/RFP/EOB2842 to J.C.S.] and the Irish Research Council's EMBARK Postgraduate Scholarship Scheme [grant number RS/2010/2147 to E.J.T.]. Additional support was provided by the US National Science Foundation through the Graduate Research Fellowship Program [to E.J.T.], and by a grant funded jointly by the Biotechnology and Biological Sciences Research Council, Department for Environment Food and Rural Affairs, Natural Environment Research Council, the Scottish Government and the Wellcome Trust, under the Insect Pollinators Initiative [BB/I000143/1 to G.A.W.]. Deposited in PMC for release after 6 months.
References
- Adler L. S. (2000). The ecological significance of toxic nectar. Oikos 91, 409-420 [Google Scholar]
- Adler L. S., Irwin R. E. (2005). Ecological costs and benefits of defenses in nectar. Ecology 86, 2968-2978 [Google Scholar]
- Adler L. S., Wink M., Distl M., Lentz A. J. (2006). Leaf herbivory and nutrients increase nectar alkaloids. Ecol. Lett. 9, 960-967 [DOI] [PubMed] [Google Scholar]
- Aizen M. A., Harder L. D. (2007). Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88, 271-281 [DOI] [PubMed] [Google Scholar]
- Baker H. G. (1975). Sugar concentrations in nectars from hummingbird flowers. Biotropica 7, 37-41 [Google Scholar]
- Baker H. G. (1977). Non-sugar chemical constituents of nectar. Apidologie (Celle) 8, 349-356 [Google Scholar]
- Baker H., Baker I. (1975). Studies of nectar-constitution and pollinator–plant coevolution. In Coevolution of Animals and Plants (ed. Gilbert L. E., Raven P. H.), pp. 591-600 Austin, TX: University of Texas Press; [Google Scholar]
- Baker H., Opler P., Baker I. (1978). A comparison of the amino acid complements of floral and extrafloral nectars. Bot. Gaz. 139, 322-332 [Google Scholar]
- Burke J. W., Doskotch R. W. (1990). High field 1H- and 13C-NMR assignments of grayanotoxins I, IV and XIV isolated from Kalmia angustifolia. J. Nat. Prod. 53, 131-137 [DOI] [PubMed] [Google Scholar]
- Chittka L., Dyer A. G., Bock F., Dornhaus A. (2003). Psychophysics: bees trade off foraging speed for accuracy. Nature 424, 388 [DOI] [PubMed] [Google Scholar]
- Després L., David J. P., Gallet C. (2007). The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 22, 298-307 [DOI] [PubMed] [Google Scholar]
- Detzel A., Wink M. (1993). Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 4, 8-18 [Google Scholar]
- Dunipace L., Meister S., McNealy C., Amrein H. (2001). Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 11, 822-835 [DOI] [PubMed] [Google Scholar]
- Ehlers B., Olesen J. (1997). The fruit-wasp route to toxic nectar in Epipactis orchids. Flora 192, 223-229 [Google Scholar]
- Gill R. J., Ramos-Rodriguez O., Raine N. E. (2012). Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning J. I. (2002). How do herbivorous insects cope with noxious secondary plant compounds in their diet? Entomol. Exp. Appl. 104, 15-25 [Google Scholar]
- Goulson D., Peat J., Stout J. C., Tucker J., Darvill B., Derwent L. C., Hughes W. O. H. (2002). Can alloethism in workers of the bumblebee, Bombus terrestris, be explained in terms of foraging efficiency? Anim. Behav. 64, 123-130 [Google Scholar]
- Hagler J., Buchmann S. (1993). Honey bee (Hymenoptera: Apidae) foraging responses to phenolic-rich nectars. J. Kansas Entomol. Soc. 66, 223-230 [Google Scholar]
- Heil M. (2011). Nectar: generation, regulation and ecological functions. Trends Plant Sci. 16, 191-200 [DOI] [PubMed] [Google Scholar]
- Heinrich B. (2004). Bumblebee Economics. Cambridge, MA: Harvard University Press; [Google Scholar]
- Henry M., Béguin M., Requier F., Rollin O., Odoux J. F., Aupinel P., Aptel J., Tchamitchian S., Decourtye A. (2012). A common pesticide decreases foraging success and survival in honey bees. Science 336, 348-350 [DOI] [PubMed] [Google Scholar]
- Hill C. A., Fox A. N., Pitts R. J., Kent L. B., Tan P. L., Chrystal M. A., Cravchik A., Collins F. H., Robertson H. M., Zwiebel L. J. (2002). G protein-coupled receptors in Anopheles gambiae. Science 298, 176-178 [DOI] [PubMed] [Google Scholar]
- Janzen D. H. (1977). Why don't ants visit flowers? Biotropica 9, 252 [Google Scholar]
- Khoury D. S., Myerscough M. R., Barron A. B. (2011). A quantitative model of honey bee colony population dynamics. PLoS ONE 6, e18491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmar J. A., Baumann T. W. (1999). Caffeine in citrus flowers. Phytochemistry 52, 19-23 [Google Scholar]
- London-Shafir I., Shafir S., Eisikowitch D. (2003). Amygdalin in almond nectar and pollen – facts and possible roles. Plant Syst. Evol. 238, 87-95 [Google Scholar]
- Manson J. S., Otterstatter M. C., Thomson J. D. (2010). Consumption of a nectar alkaloid reduces pathogen load in bumble bees. Oecologia 162, 81-89 [DOI] [PubMed] [Google Scholar]
- Michener C. D. (1974). The Social Behaviour of the Bees: A Comparative Study. Cambridge, MA: Harvard University Press (Belknap Press) [Google Scholar]
- Mommaerts V., Wäckers F., Smagghe G. (2013). Assessment of gustatory responses to different sugars in harnessed and free-moving bumblebee workers (Bombus terrestris). Chem. Senses 38, 399-407 [DOI] [PubMed] [Google Scholar]
- Müller C. B., Schmid-Hempel P. (1992). Correlates of reproductive success among field colonies of Bombus lucorum: the imporance of growth and parasites. Ecol. Entomol. 17, 343-353 [Google Scholar]
- Mustard J. A., Dews L., Brugato A., Dey K., Wright G. A. (2012). Consumption of an acute dose of caffeine reduces acquisition but not memory in the honey bee. Behav. Brain Res. 232, 217-224 [DOI] [PubMed] [Google Scholar]
- Nicolson S. W. (2011). Bee food: The chemistry and nutritional value of nectar, pollen and mixtures of the two. African Zoology 46, 197-204 [Google Scholar]
- Ollerton J., Winfree R., Tarrant S. (2011). How many flowering plants are pollinated by animals? Oikos 120, 321-326 [Google Scholar]
- Raguso R. A., Levin R. A., Foose S. E., Holmberg M. W., McDade L. A. (2003). Fragrance chemistry, nocturnal rhythms and pollination ‘syndromes’ in Nicotiana. Phytochemistry 63, 265-284 [DOI] [PubMed] [Google Scholar]
- Rhoades D. F., Bergdahl J. C. (1981). Adaptive significance of toxic nectar. Am. Nat. 117, 798-803 [Google Scholar]
- Robertson H. M., Wanner K. W. (2006). The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res. 16, 1395-1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. M., Warr C. G., Carlson J. R. (2003). Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 100 Suppl. 2, 14537-14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal G. A., Berenbaum M. R. (1992). Herbivores: Their Interactions with Secondary Plant Metabolites. New York, NY: Academic Press; [Google Scholar]
- Roubik D. W. (2002). Tropical agriculture: the value of bees to the coffee harvest. Nature 417, 708 [DOI] [PubMed] [Google Scholar]
- Schuler M. A. (2011). P450s in plant–insect interactions. Biochim. Biophys. Acta 1814, 36-45 [DOI] [PubMed] [Google Scholar]
- Scott K., Brady R., Jr, Cravchik A., Morozov P., Rzhetsky A., Zuker C., Axel R. (2001). A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 104, 661-673 [DOI] [PubMed] [Google Scholar]
- Sellier M.-J., Reeb P., Marion-Poll F. (2011). Consumption of bitter alkaloids in Drosophila melanogaster in multiple-choice test conditions. Chem. Senses 36, 323-334 [DOI] [PubMed] [Google Scholar]
- Shields V. D., Smith K. P., Arnold N. S., Gordon I. M., Shaw T. E., Waranch D. (2008). The effect of varying alkaloid concentrations on the feeding behavior of gypsy moth larvae, Lymantria dispar (L.) (Lepidoptera: Lymantriidae). Arthropod Plant Interact. 2, 101-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravelan N., Inbar M., Ne'eman G., Distl M., Wink M., Izhaki I. (2006). The effects of nectar-nicotine on colony fitness of caged honeybees. J. Chem. Ecol. 32, 49-59 [DOI] [PubMed] [Google Scholar]
- Stephenson A. G. (1982). Iridoid glycosides in the nectar of Catalpa speciosa are unpalatable to nectar thieves. J. Chem. Ecol. 8, 1025-1034 [DOI] [PubMed] [Google Scholar]
- Stout J. C., Parnell J. A. N., Arroyo J., Crowe T. P. (2006). Pollination ecology and seed production of Rhododendron ponticum in native and exotic habitats. Biodivers. Conserv. 15, 755-777 [Google Scholar]
- Tadmor-Melamed H., Markman S., Arieli A., Distl M., Wink M., Izhaki I. (2004). Limited ability of Palestine sunbirds Nectarinia osea to cope with pyridine alkaloids in nectar of tree tobacco Nicotiana glauca. Funct. Ecol. 18, 844-850 [Google Scholar]
- Tan K., Wang Z., Yang M., Fuchs S., Luo L., Zhang Z., Li H., Zhuang D., Yang S., Tautz J., et al. (2012). Honeybees (Apis cerana) modulate dance communication in response to nectar toxicity and demand. Anim. Behav. 84, 1589-1594 [Google Scholar]
- Thomson J. D., Goodell K. (2001). Pollen removal and deposition by honeybee and bumblebee visitors to apple and almond flowers. J. Appl. Ecol. 38, 1032-1044 [Google Scholar]
- Whitehorn P. R., O'Connor S., Wackers F. L., Goulson D. (2012). Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351-352 [DOI] [PubMed] [Google Scholar]
- Wink M. (1993). Allelochemical properties or the raison d'etre of alkaloids. In The Alkaloids: Chemistry and Pharmacology, Vol. 43 (ed. Cordell G. A.), pp. 1-118 Boston, MA: Academic Press; [Google Scholar]
- Wright G. A., Mustard J. A., Simcock N. K., Ross-Taylor A. A. E., McNicholas L. D., Popescu A., Marion-Poll F. (2010). Parallel reinforcement pathways for conditioned food aversions in the honeybee. Curr. Biol. 20, 2234-2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. A., Baker D. D., Palmer M. J., Stabler D., Mustard J. A., Power E. F., Borland A. M., Stevenson P. C. (2013). Caffeine in floral nectar enhances a pollinator's memory of reward. Science 339, 1202-1204 [DOI] [PMC free article] [PubMed] [Google Scholar]