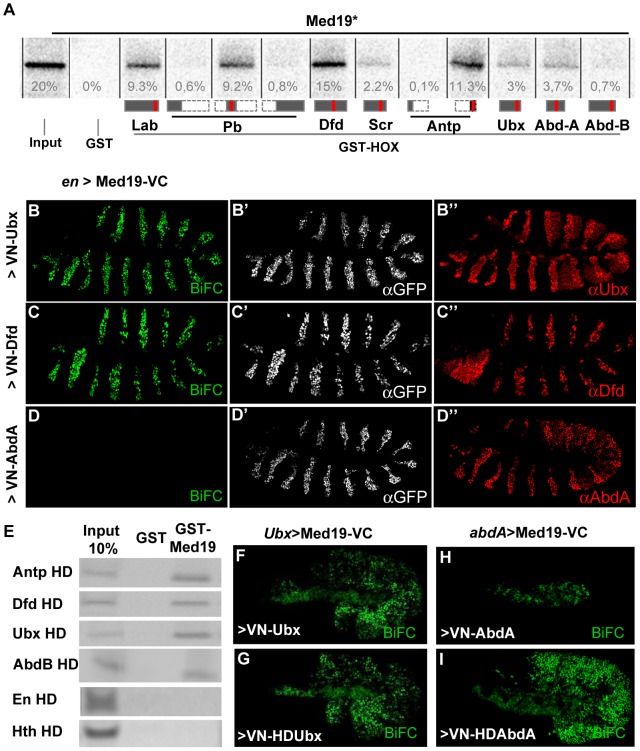
Figure 1. Hox proteins bind Med19 through their homeodomains in vitro and in vivo.
(A) GST-pulldown binding assays of 35S-Med19 to immobilized GST-Hox fusions containing full length or protein fragments (below each lane: rectangles represent the entire protein; portions present in GST-Hox chimeric proteins are black, except the HD, represented in red). (B–D) BiFC assays were carried out co-expressing Med19-VC with VN-Ubx (B), VN-Dfd (C) or VN-AbdA (D), from UAS constructs under engrailed-Gal4 control (en>). Med19-VC accumulation, detected with antibody against the GFP C-terminal region, is similar in all tests (B′–D′). Gal4-driven Hox protein accumulation is comparable to endogenous, as detected with Ubx, Dfd and AbdA specific antibodies (B″–D″). Relative BiFC fluorescent signals were quantified as in [32]. VN-Ubx signal (B) and VN-Dfd (C) yielded serial rows of nuclear fluorescence; VN-AbdA (D) gave no detectable signal. (E) Direct homeodomain binding to Med19. Pulldowns with immobilized GST or GST-Med19 employed 70 aa-long 35S-labelled peptides centered on the HDs of Antp, Dfd, Ubx, AbdB, En and Hth. (F–I) Direct homeodomain binding to Med19 in BiFC assay. Co-expression of Med19-VC with VN-Ubx (F), or with its HD (VN-HDUbx; G), under Ubx-Gal4 control gives indistinguishable BiFC signals. Expression of Med19-VC under abdA-Gal4 control yielded no fluorescence with VN-AbdA (H) but gave a strong signal with VN-HDAbdA alone (I).