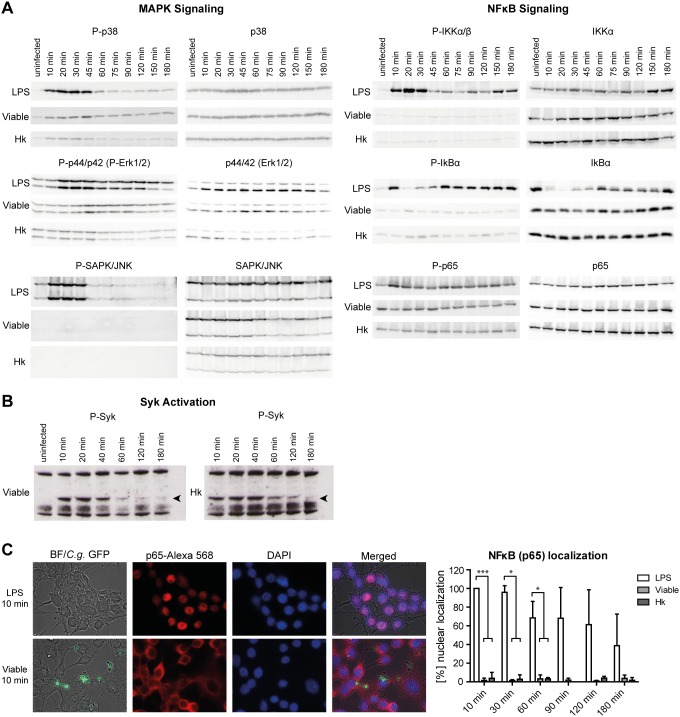
Figure 3. C. glabrata does not induce MAP-kinase or NFκB signaling cascades upon phagocytosis but activates Syk.
RAW264.7 macrophages were stimulated with LPS (1 µg/ml) or infected with viable or heat killed (Hk) C. glabrata (MOI of 5) for indicated time points. (A) Cell lysates were subjected to Western Blot analyses by using antibodies detecting either the phosphorylated or unphosphorylated form (as a loading control) of p38, p44/42 (Erk1/2), SAPK/JNK, IKKαβ, IκBα and p65. Only LPS treatment induced changes in phosphorylation patterns of analyzed proteins. Data shown are representatives of three independent experiments. (B) Cell lysates were resolved on SDS-PAGE and membranes blotted for phosphorylated Syk (P-Syk) as described in [25] (C) Localization of the NFκB subunit p65 was analyzed by immunofluorescence microscopy. Representative pictures of macrophages treated with LPS or viable C. glabrata for 10 min are shown on the left site, a quantification of indicated time points on the right site. Percentage of NFκB nuclear localization was quantified for all macrophages (LPS) or for yeast-bound macrophages (viable, heat killed). While LPS induced the translocation of p65 to the nucleus, C. glabrata independent of its viability, did not. Statistical analysis was performed for C. glabrata-infected versus LPS-treated macrophages at the indicated time points (n≥3; *p<0.05, ***p<0.005 by unpaired Student’s t test).