Table 1.
Optimization results for the cross-coupling of 2-chloropyridine (1a) with ethyl 4-bromobutyrate (2a).a
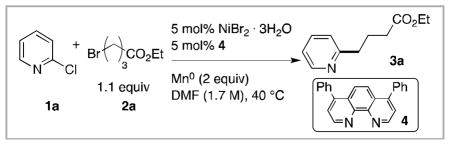
| ||
|---|---|---|
| Entry | Change from above conditions | Yield (%)b |
| 1 | None | 82 |
| 2 | 10 mol% NiBr2•3H2O/4 | 74c |
| 3 | 1 equiv each 1a and 2a | 78 |
| 4 | 1.1 equiv 1a | 71 |
| 5 | 1,10-phenanthroline (5) in place of 4 | 64 |
| 6 | 4,4′-di-t-butyl-2,2′-bipyridine (6) in place of 4 | 66 |
| 7 | 4,4′-di-methoxy-butyl-2,2′-bipyridine (7) in place of 4 | 69 |
| 8 | 4,4′,4″-tri-tert-butyl-2,2′,:6′,2″-terpyridine (8) in place of 4 | 15 |
| 9 | NiCl2(glyme) in place of NiBr2•3H2O | 79 |
| 10 | Reaction run at 20 °C | 55c |
| 11 | Reaction run at 60 °C | 70c |
| 12 | Reaction run at 80 °C | 62c |
| 13 | 25% DMA in THF in place of DMF | 15d |
| 14 | Zn0 (<10 μm) in place of Mn0 | 19e |
| 15 | Al0/PbBr2 in place of Mn0 | 2e,g |
Reaction conditions: DMF (1 mL), NiBr2•3H2O (0.15 mmol), 1a (3.00 mmol), 2a (3.30 mmol), ligand (0.15 mmol), and Mn0 (6.00 mmol) were added to a 1 dram vial on the bench top and heated under air for 4–22 h.
GC yield corrected vs. dodecane internal standard.
Isolated yield.
Observed partial conversion of starting material at 24 h.
Major coupled product was the alkyl dimer.
No reaction of 2a was observed.
