Figure 1. The GST-E4-ORF1 fusion protein binds specifically and directly to the functional PI3K p85:p110 heterodimer in an in vitro pulldown assay.
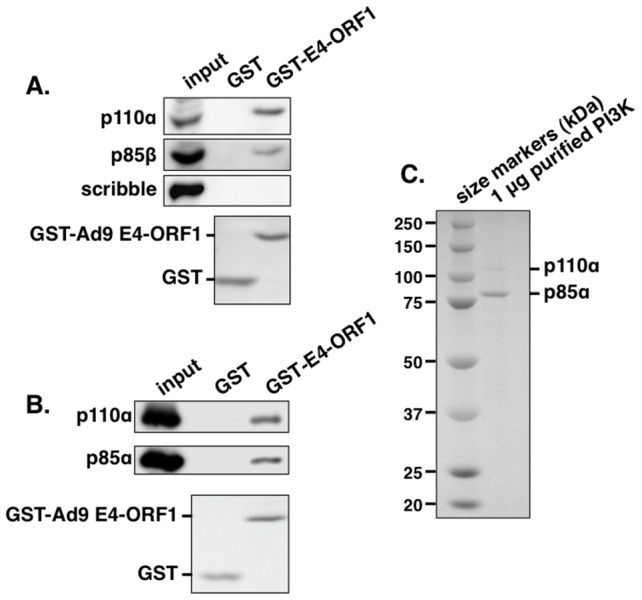
(A) The GST-E4-ORF1 protein binds to the endogenously expressed p85 regulatory and p110 catalytic PI3K subunits in MCF10A cell lysates. MCF10A cell extract (500 µg of protein) was subjected to pulldown assays with the indicated GST fusion protein. Recovered proteins, as well as cell extract (input), were analyzed in an immunoblot assay with antibodies to the indicated proteins. Coomassie Brilliant Blue staining of the lower portion of the protein gel verified use of an equivalent amount of GST and GST-Ad9 E4-ORF1 protein. (B) The GST-E4-ORF1 protein also binds to purified, functional recombinant human PI3K. Purified, recombinant human PI3K protein (1 µg; >90% pure full-length p85α and p110α) (see Materials and Methods ) was subjected to a pulldown assay with either control GST or GST-Ad9 E4-ORF1 protein. Recovered proteins and 1/2 of input PI3K protein were analyzed in an immunoblot assay with p85α and p110α antibodies. Coomassie Brilliant Blue staining of the lower portion of the protein gel verified use of an equivalent amount of GST and GST-Ad9 E4-ORF1 protein in the assay. (C) Verification of the purity of the recombinant PI3K protein. The input PI3K protein (1 µg) used in Figure 1B was resolved by SDS-PAGE, and the protein gel was stained with Coomassie Brilliant Blue. The recombinant p85 protein is present in slight molar excess over the recombinant p110 protein in the purified PI3K preparation.
