Figure 2.
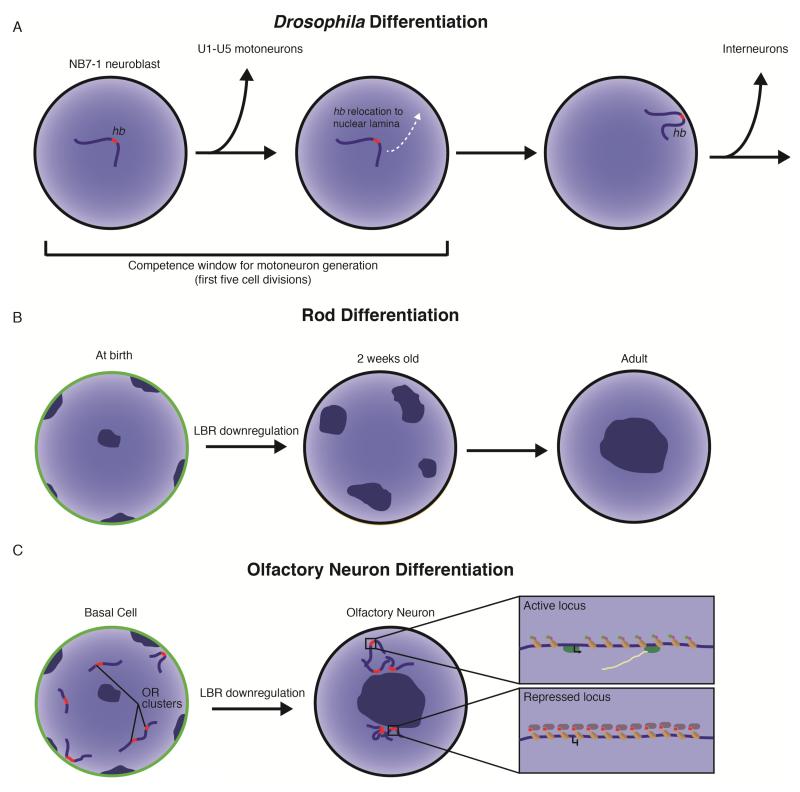
Nuclear architecture in neurogenesis. A. During Drosophila neurogenesis, relocation of the hunchback (hb) gene marks the end of a competence window where neuroblast NB7-1 can be induced to generate U1/U2 motoneurons by ectopic hb expression. Prior to its first five cell divisions, NB7-1 generates U1-U5 motoneurons sequentially, but ectopic expression of hb can led to additional neurons with U1/U2 identity. After five cell divisions, the hb gene transitions from the nuclear interior to associate with the nuclear lamina, where it is stably silenced and can not promote U1/U2 identity. NB7-1 gives rise to interneurons after hb is recruited to the nuclear lamina. B. At birth, the nuclei of rod photoreceptor cells in nocturnal animals demonstrate conventional organization with chromocenters (dark blue regions) at the nuclear periphery and expression of Lamin B receptor (LBR, green outline). As these cells mature, LBR is downregulated and the chromocenters gradually fuse to leave a single centrally-located chromocenter in the nuclear interior. C. In undifferentiated basal cells, olfactory receptor (OR) genes are dispersed through the nucleus and expression of LBR is robust. In mature olfactory sensory neurons (OSNs), OR genes aggregate near a centrally located chromocenter as LBR expression is lost. Boxes highlight the local chromatin of active and repressed OR alleles. The active OR allele loops out into a nuclear domain of active chromatin and is marked by H3K4me3 (green circles), histone acetylation (purple circles), and transcribing RNA polymerase. The repressed OR genes are enriched for H3K9me3 (red circles) and heterochromatin protein 1β (purple ovals).