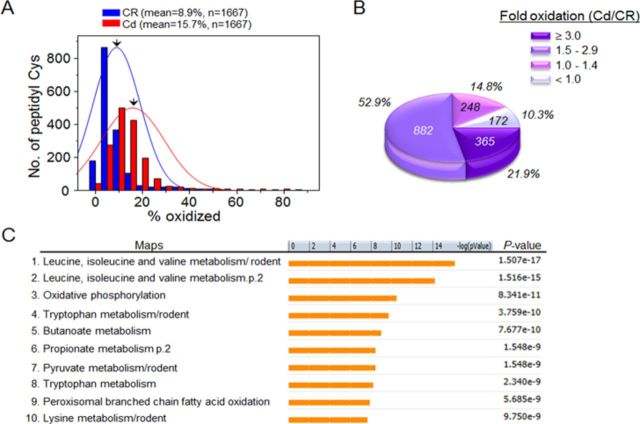
FIG. 4.
Cd-induced oxidation in liver mitochondrial redox proteome. Freshly isolated liver mitochondria (120 μg protein) obtained from saline CR (3 mice) and Cd (3 mice) were analyzed for redox proteomics using redox ICAT-based mass spectrometry (MS). (A) Distribution of the oxidized states (% oxidized) of the Cys-including peptide (peptidyl Cys) relevant to 655 proteins from CR (blue; n = 1667) and Cd (red; n = 1667). The mean value of % oxidation from 1667 peptidyl Cys from CR and Cd were 8.9% and 15.7%, respectively. (B) Pie chart showing the distribution of peptidyl Cys according to the measured fold oxidation. Of 1667 peptidyl Cys, 77% were oxidized more than 50% (1.5-fold) by Cd treatment. (C) MetaCore Bioinformatics software identified significant pathway maps that are regulated by Cd-oxidized peptidyl Cys (fold oxidation ≥ 1.5, n = 1247). Of the 86 statistically significant pathways, the top 10 pathways are shown (C).