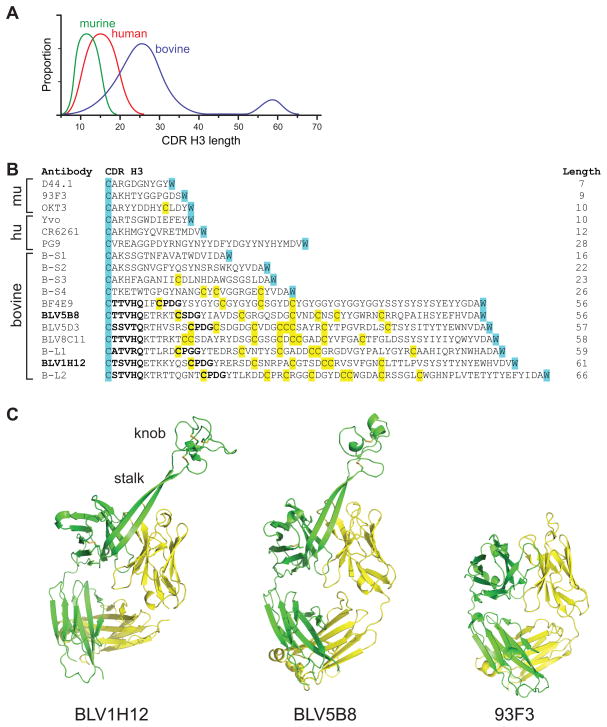
Figure 1. Identification of a new structural domain in bovine antibodies.
(A) Comparison of CDR H3 length amongst murine, human, and bovine repertoires. An ultralong subset of over 60 amino acids is uniquely found in bovine heavy chains (blue). (B) Sequences of representative CDR H3 from murine (mu), human (hu), or bovine sequences from the literature along with six bovine sequences (B-S1 to B-S4, and B-L1 and B-L2) from our sequencing results. The conserved cysteine of framework 3 and tryptophan of framework 4 that define CDR H3 boundaries in all antibody variable regions are highlighted in cyan for reference, and cysteines are yellow. The lengths of the CDR H3s are indicated at the right. The murine antibodies include D44.1, an anti-HEL antibody, 93F3, an aldolase, and OKT3, a therapeutic antibody targeting human CD3. This antibody is unusual in having a free cysteine in CDR H3. The human antibodies include Yvo, a cryoglobulin, CR6261, an anti-influenza A hemaglutinin, and PG9, an anti-HIV antibody which has one of the longest human CDR H3s. The bovine antibodies represent the ultralong sequences in the literature, and short sequences for comparison. BLV5B8 and BLV1H12 (indicated in bold) were used in our structure determinations. Relatively conserved TTVHQ and CPDG motifs are in bold. (C) Crystal structures of BLV1H12 (left) and BLV5B8 (middle) Fabs compared to the 93F3 Fab with a “normal” CDR H3 (right). A superlong, two β-stranded stalk protrudes from each bovine VH immunoglobulin domain and terminates in an unusual three disulfide-linked knob domain. See also Figure S1 and Table S1.