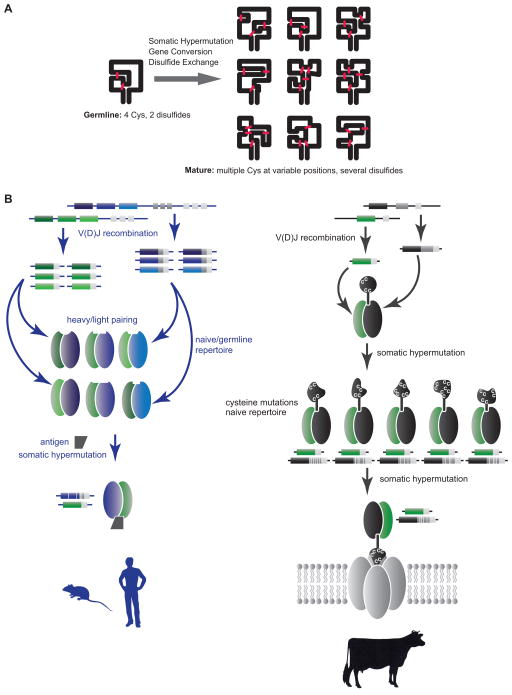
Figure 7. Model for ultralong CDR H3 diversification into novel minifolds.
(A) A schematic of the DH2 knob with 4 cysteines is shown on the left, with SH and/or gene conversion leading to a multitude of new cysteine patterns and new loops on the right. (B) Mechanisms for generating antibody diversity. In humans and mice (left), combinatorial diversity through V(D)J recombination and VH-VL pairing creates a multitude of different binding sites, which are further optimized following antigen exposure by somatic hypermutation. In cows (right), combinatorial diversity is severely limited; however, somatic mutation to and from cysteines can reshape the “knob” region, creating substantial structural diversity in ultralong CDR H3s. These antibodies may be further optimized through SH and may bind unique targets such as pores or channels. See also Figure S5 and Table S6.