Abstract
Objective
To identify differentially expressed salivary proteins in bisphosphonate-related osteonecrosis of the jaw (BRONJ) patients that could serve as biomarkers for BRONJ diagnosis.
Subjects and Methods
Whole saliva obtained from 20 BRONJ patients and 20 controls were pooled within groups. The samples were analyzed using iTRAQ-labeled two-dimensional liquid chromatography-tandem mass spectrometry.
Results
Overall, 1340 proteins were identified. Of these, biomarker candidates were selected based on p-value (<0.001), changes in protein expression (≥1.5-fold increase or decrease), and unique peptides identified (≥2). Three comparisons made between BRONJ and controls identified 200 proteins to be differentially expressed in BRONJ patients. A majority of these proteins were predicted to have a role in drug metabolism, immunological and dermatological diseases. Of all the differentially expressed proteins, we selected metalloproteinase-9 and desmoplakin for further validation. Immunoassays confirmed increased expression of metalloproteinase-9 in individual saliva (p=0.048) and serum samples (p=0.05) of BRONJ patients. Desmoplakin was undetectable in saliva. However, desmoplakin levels tended to be lower in BRONJ serum than controls (p=0.157).
Conclusions
Multiple pathological reactions are involved in BRONJ development. One or more proteins identified by this study may prove to be useful biomarkers for BRONJ diagnosis. The role of metalloproteinase-9 and desmoplakin in BRONJ requires further investigation.
Keywords: Bisphosphonate; Osteonecrosis of the Jaw; Bisphosphonate-related osteonecrosis of the jaw; Proteomics; Saliva, Biological markers, Biomarkers
Introduction
Long-term bisphosphonate (BP) therapy is associated with bisphosphonate-related osteonecrosis of the jaw (BRONJ) (Hoff et al. 2008, Ruggiero et al. 2004). To date, the etiology and risk factors that contribute to BRONJ pathogenesis are largely unknown. Current diagnostic tools to assess skeletal health in patients receiving BP therapy are limited to imaging techniques, including computerized tomography (CT) and cone-beam computerized tomography (CBCT) (Bedogni et al. 2008, Treister et al. 2010). These methods rely on changes in the physical characteristics of bone, which minimizes the likelihood of identifying “at risk” patients before they develop clinical signs of BRONJ. Therefore, it is important to identify biomarkers that precede the onset of BRONJ lesions. Since long-term BP administration is suggested to impair bone remodeling and angiogenesis-related processes (Woo et al. 2006, Yin et al. 2011), mediators of bone turnover and angiogenesis are rationale candidates to evaluate as BRONJ biomarkers. Several cross-sectional studies have attempted to investigate the predictive value of bone turnover markers (e.g., C-terminal telopeptide (CTX), N-terminal telopeptide (NTX), osteocalcin, alkaline phosphatase) and angiogenesis markers (e.g., vascular endothelial growth factor (VEGF)) in BRONJ development (Lazarovici et al. 2010, Lehrer et al. 2009, Marx et al. 2007, Vincenzi et al. 2012). Currently, no scientific evidence exists to support the use of these markers in assessing BRONJ risk (Hellstein et al. 2011).
Recently, patient-based proteomic studies have become valuable approaches in discovering biomarkers for various diseases (Xie et al. 2008). In this study, we utilized an advanced quantitative proteomics approach to identify differentially expressed salivary proteins in BRONJ patients that can serve as potential biomarkers for BRONJ diagnosis.
Material and Methods
Subjects
The University of Minnesota and Park Nicollet Institutional Review Boards approved this study and written informed consent was obtained from all participants. Cancer patients above 30 years of age who had received at least 10 infusions of intravenous BP's for cancer management were eligible for this study. Patients with a history of radiation therapy to the head and neck region or neoplasms (including metastasis of distant cancers) involving the head and neck region were excluded. The study population included 20 patients who developed BRONJ following intravenous BP therapy and 20 control patients who did not develop BRONJ following intravenous BP therapy. The American Association of Oral and Maxillofacial Surgeons criteria (Ruggiero et al. 2009) was used to define BRONJ: “exposed bone in the maxillofacial area occurring in the absence of head and neck irradiation and showing no evidence of healing for at least 8 weeks after identification in patients treated with BP therapy”. Controls were defined as patients receiving intravenous BP therapy with no clinical or radiographic evidence of necrotic bone, including non-specific symptoms seen in Stage 0 BRONJ patients.
Saliva Sample Collection and Processing
Unstimulated whole saliva (5mL) was collected from BRONJ and control subjects by having each subject swallow and then expectorate continuously into 50-ml sterile, polypropylene conical tube for a period of 5 to 15 minutes. Samples were centrifuged at 2,600g for 15 minutes at 4°C. The supernatant containing the soluble fraction of salivary proteins was aliquoted into 1mL tubes, mixed with protease inhibitors (courtesy of David T. Wong, UCLA School of Dentistry, CA) and stored at-80°C.
Protein Digestion, iTRAQ Labeling, and Peptide Fractionation
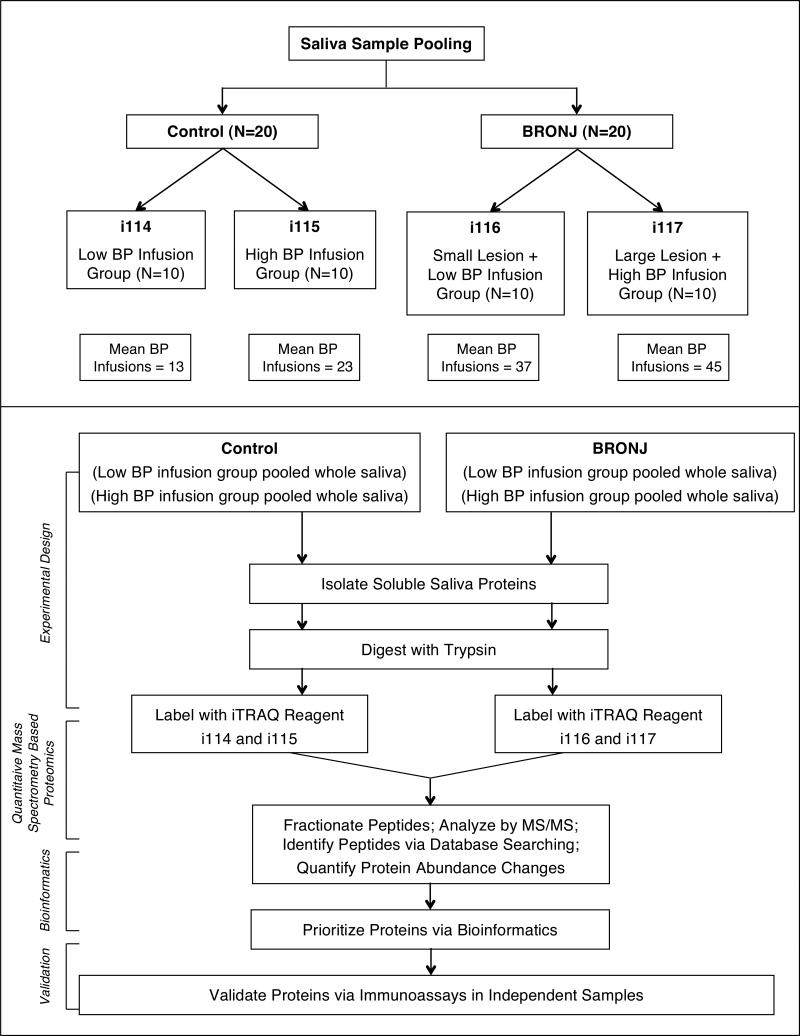
Total protein concentration in the soluble fraction of saliva was quantified using the BCA assay (Thermo Pierce). Based on BRONJ lesion size, BRONJ subjects were characterized into “large lesion” (≥10mm) and “small lesion” (<10mm) groups. Notably, patients in the large lesion group had received higher number of BP infusions (mean=45) compared to small lesion BRONJ group (mean=37). Control subjects were categorized into “high” and “low” infusion groups based on whether they had received higher or lower BP infusions than the median number of infusion for all control patients (median=16). Pooled samples (N=10) were created for each of the four subgroups using 10 μg of protein from each subject (Figure 1). Proteins in each pooled sample were digested overnight with trypsin according to filter-aided sample preparation (FASP) protocol (Wisniewski et al. 2009). Resulting peptides were concentrated and purified via reversed phase solid-phase extraction columns (Waters) and later labeled with the iTRAQ reagent (Applied Biosystems, Foster City) (Ross et al. 2004). Subsequently, the iTRAQ-labeled peptide mixtures were combined and fractionated using strong cation exchange (SCX) chromatography (Bandhakavi et al. 2011). Fractions were analyzed by reversed-phase microcapillary liquid chromatography mass spectrometry (Xie et al. 2008).
Figure 1.
BRONJ Biomarker Discovery Strategy.
Mass Spectrometry
Mass spectrometry was performed using a linear ion trap-Orbitrap (LTQ-Orbitrap) Velos instrument (Thermo Fisher Scientific) (Olsen et al. 2009). The LTQ-Orbitrap Velos was operated in a top-ten data dependent mode using survey scans at 30,000 resolution from 300 to 1800 m/z. Tandem MS scans were acquired with an isolation width of 2 m/z and higher energy collisional dissociation (HCD) fragmentation mode with 40% normalized collision energy for 20 milliseconds. The automatic gain control settings were 3 × 105 ions in the ion trap and 1 × 106 in the Orbitrap. Dynamic exclusion was used with duration of 15 seconds and a repeat count of 1.
Protein Identification and Quantification
Raw files were converted to mzXml using msconvert (distributed as part of ProteoWizard 1.6.1260). Tandem mass spectra were searched against a human database including scrambled sequences and common contaminant proteins (136002 entries) using Sequest v27.0. Search parameters included a 1.6 amu (atomic mass units) precursor and 0.8 amu fragment mass tolerance, 2 missed cleavages, partial trypsin specificity, fixed modifications of cysteine acetamidylation, iTRAQ reagent at lysines and N-termini, and variable modification of methionine oxidation. Search results were filtered to 99% protein probability and 95% peptide probability in Scaffold (v3.3.1, Proteome Software), producing false discovery rates of 0.8–3.6%. Proteins were quantified using customized software developed by us (Onsongo et al. 2010) and biological meaning of differentially expressed proteins was assessed via bioinformatics analysis using the Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, Inc). All computational hardware and software was made available via an ongoing collaboration with the Minnesota Supercomputing Institute.
Statistical Analysis
For mass spectrometry data, protein's abundance ratio and p-value were computed using open source software that implements an intensity-based weighted approach which is described elsewhere (Onsongo et al. 2010). For ELISA, differences between BRONJ and control groups were tested using a two-sided Student's t-test. A p-value of less than 0.05 was considered statistically significant.
Results
Patient Characteristics
Selected patient characteristics are listed in Table 1. Cases and controls did not differ significantly in terms of age, sex, or cancer diagnosis. All subjects had received or were receiving intravenous zoledronate, pamidronate, or both. The average number of BP infusions was significantly higher in BRONJ cases compared to controls (41.0±27.2 infusions versus 17.9±8.1; P<0.01). BRONJ cases also received BP therapy for a longer period, (mean, 44.9±27.8 months versus 31.2±16.2 months) although the difference was not statistically significant (P=0.06). Most patients had received BP therapy for metastatic breast cancer management (12 BRONJ and 17 controls, Table 1).
Table 1. Baseline patient characteristics (N=40).
• Plus–minus values are means ±SD
| Characteristic | BRONJ (N=20) | Non-BRONJ (N=20) | P † |
|---|---|---|---|
| Age - years | 64.3±10.1 | 62.4±11.3 | 0.58 |
| Sex – no. (%) | 0.27 | ||
| Male | 7 (35) | 3 (15) | |
| Female | 13 (65) | 17 (85) | |
| Malignant Disease – no. (%) | 0.42 | ||
| Breast cancer | 12 (60) | 17 (85) | |
| Multiple myeloma | 2 (10) | 2 (10) | |
| Prostate cancer | 1 (5) | 1 (5) | |
| Lung cancer | 2 (10) | 0 (0) | |
| Renal cell carcinoma | 2 (10) | 0 (0) | |
| Others | 1 (5) | 0 (0) | |
| Type of Bisphosphonates – no. (%) | 0.28 | ||
| Zoledronate | 14 (70) | 18 (90) | |
| Pamidronate | 2 (10) | 0 (0) | |
| Pamidronate + Zoledronate | 4 (20) | 2 (10) | |
| Bisphosphonate infusions | |||
| All cancer | 41.0±27.2 | 17.9±8.1 | <0.01 |
| Duration of Bisphosphonate exposure – in months | 44.9±27.8 | 31.2±16.2 | 0.06 |
| All cancer |
• P-values were calculated from a Fisher's-exact test or a t-test for categorical or continuous variables, respectively.
At the time of saliva sampling, BRONJ cases had discontinued BP therapy for an average of 9.3 months (range 2 to 27 months) following initial BRONJ diagnosis, while controls continued to receive BP therapy for cancer management.
BRONJ Characteristics
BRONJ was noted most frequently in the posterior region of the mandible (75%). Most patients (80%) developed BRONJ following dentoalveolar procedures, while some (20%) developed it spontaneously without an obvious precipitating event. Pain was the most common symptom in BRONJ cases, with some exhibiting evidence of infection. As part of BRONJ management, most BRONJ cases were using antibiotics (50%) or a chlorhexidine mouth rinse (82%) at the time of study enrolment.
Analysis of Salivary Proteome
Using proteomic profiling, we identified 1340 proteins. Of these proteins, biomarker candidates were selected based on p-value (<0.001), the magnitude of change in protein expression (1.5-fold or greater increase or decrease) relative to the respective comparison group (e.g., comparing high infusion cases versus high infusion controls), and unique peptides identified (two or more).
Three comparisons were made between BRONJ and control groups: (I) low-infusion controls versus low-infusion BRONJ (i114 vs i116); (II) high-infusion controls versus high-infusion BRONJ (i115 vs i117); and (III) high-infusion controls versus low-infusion BRONJ (i115 vs i116). In order to have a proximate comparison between cases and controls based on number of BP infusions, we analyzed high infusion control group with low infusion BRONJ group. Two hundred proteins were found to be differentially expressed in BRONJ cases compared to controls, including 15 proteins in comparison-I (Table 2), 78 proteins in comparison-II (Table 3), and 107 proteins in comparison-III (Table 4).
Table 2.
Differentially expressed salivary proteins in BRONJ cases compared to controls in the low BP infusion groups
| Accession Number | Unique Peptides | Protein Name | Fold Change† | P value |
|---|---|---|---|---|
| Low BP Infusion BRONJ Group (i116) vs Low BP Infusion Control Group (i114) | ||||
| 075556 | 4 | Mammaglobin-B | ⇑ 13.885 | 8.71E-08 |
| C9JRG0 | 2 | Hemoglobin, delta | ⇑ 7.914 | 1.16E-06 |
| P69892 | 2 | Hemoglobin subunit gamma-2 | ⇑ 5.308 | 5.91E-04 |
| Q04118 | 3 | Basic salivary proline-rich protein 3 | ⇑ 2.408 | 3.54E-04 |
| P00918 | 4 | Carbonic anhydrase 2 | ⇑ 2.230 | 6.84E-06 |
| P34096 | 2 | Ribonuclease 4 | ⇑ 1.722 | 7.56E-08 |
| P80723 | 5 | Brain acid soluble protein 1 | ⇑ 1.660 | 6.59E-04 |
| P25705 | 3 | ATP synthase subunit alpha, mitochondrial | ⇑ 1.547 | 1.35E-04 |
| P05546 | 4 | Heparin cofactor 2 | ⇑ 1.502 | 8.06E-09 |
| C9JMC5 | 4 | Aldehyde dehydrogenase 3 family, member A1 | ⇓ 2.310 | 3.11E-15 |
| P09211 | 2 | Glutathione S-transferase P | ⇓ 2.123 | 5.46E-07 |
| O60218 | 6 | Aldo-keto reductase family 1 member B10 | ⇓ 1.850 | 2.41E-09 |
| P55786 | 4 | Puromycin-sensitive aminopeptidase | ⇓ 1.622 | 1.29E-06 |
| P06731 | 2 | Carcinoembryonic antigen-related cell adhesion molecule 5 | ⇓ 1.572 | 3.73E-04 |
| Q5W0H4 | 2 | Tumor protein, translationally-controlled 1 | ⇓ 1.557 | 7.70E-04 |
⇑ Represents upregulation and ⇓ Represents downregulation
Table 3.
Differentially expressed salivary proteins in BRONJ cases compared to controls in the high BP infusion groups
| Accession Number | Unique Peptides | Protein Name | Fold Change† | P value |
|---|---|---|---|---|
| High BP Infusion BRONJ Group (i117) vs High BP Infusion Control Group (i115) | ||||
| Q53FA3 | 2 | Heat shock 70kDa protein 1-like | ⇑ 4.099 | 8.65E-05 |
| C9JRG0 | 2 | Hemoglobin, delta | ⇑ 3.206 | 6.24E-07 |
| F5H3W7 | 20 | Matrix metallopeptidase 9 | ⇑ 2.903 | 1.95E-04 |
| E7EW61 | 5 | Transthyretin | ⇑ 2.870 | 3.09E-10 |
| P02763 | 9 | Alpha-1-acid glycoprotein 1 | ⇑ 2.848 | 1.90E-05 |
| P14780 | 2 | Matrix metalloproteinase-9 | ⇑ 2.806 | 5.48E-09 |
| P01857 | 23 | Ig gamma-1 chain C region | ⇑ 2.715 | 3.36E-04 |
| P80419 | 2 | Ig heavy chain V-III region GAR | ⇑ 2.656 | 1.98E-05 |
| P05546 | 4 | Heparin cofactor 2 | ⇑ 2.601 | 2.97E-13 |
| P01009-2 | 3 | Isoform 2 of Alpha-1-antitrypsin | ⇑ 2.600 | 6.78E-14 |
| 015144 | 2 | Actin-related protein 2/3 complex subunit 2 | ⇑ 2.361 | 1.39E-14 |
| P29622 | 3 | Kallistatin | ⇑ 2.335 | 3.56E-04 |
| P02652 | 6 | Apolipoprotein A-II | ⇑ 2.285 | 1.90E-05 |
| Q9H9S4 | 2 | Calcium-binding protein 39-like | ⇑ 2.242 | 2.96E-10 |
| P01880 | 2 | Ig delta chain C region | ⇑ 2.148 | 2.89E-15 |
| P63267 | 3 | Actin, gamma-enteric smooth muscle | ⇑ 2.115 | 4.13E-07 |
| P01031 | 4 | Complement C5 | ⇑ 2.104 | 1.44E-15 |
| P35542 | 2 | Serum amyloid A-4 protein | ⇑ 2.102 | 7.91E-04 |
| P04217 | 4 | Alpha-1B-glycoprotein | ⇑ 2.062 | 4.24E-04 |
| P61586 | 2 | Transforming protein RhoA | ⇑ 2.039 | 6.90E-09 |
| P01763 | 2 | Ig heavy chain V-III region WEA | ⇑ 2.034 | 2.16E-07 |
| Q9NUQ9 | 6 | Protein FAM49B | ⇑ 2.002 | 3.98E-05 |
| Q5W0X3 | 2 | FK506 binding protein 1A, 12kDa | ⇑ 1.964 | 8.65E-06 |
| Q15782 | 6 | Chitinase-3-like protein 2 | ⇑ 1.929 | 2.78E-05 |
| P35241 | 3 | Radixin | ⇑ 1.878 | 9.46E-13 |
| P62745 | 2 | Rho-related GTP-binding protein RhoB | ⇑ 1.826 | 2.11E-10 |
| D6RHI9 | 3 | Ribonuclease T2 | ⇑ 1.812 | 4.50E-07 |
| P01770 | 2 | Ig heavy chain V-III region NIE | ⇑ 1.766 | 5.58E-04 |
| P06316 | 2 | Ig lambda chain V-I region BL2 | ⇑ 1.756 | 1.17E-04 |
| P01776 | 2 | Ig heavy chain V-III region WAS | ⇑ 1.738 | 2.77E-04 |
| P00736 | 2 | Complement C1r subcomponent | ⇑ 1.721 | 9.64E-12 |
| B4DJS1 | 3 | cDNA FLJ57383, highly similar to Coronin-1A | ⇑ 1.698 | 1.48E-06 |
| C9JZD1 | 2 | Actin related protein 2/3 complex, subunit 3, 21kDa | ⇑ 1.628 | 3.61E-04 |
| O60888-2 | 2 | Isoform A of Protein CutA | ⇑ 1.620 | 8.97E-07 |
| tr | 2 | Uncharacterized protein OS=Homo sapiens PE=4 SV=1 | ⇑ 1.585 | 7.09E-04 |
| P04430 | 3 | Ig kappa chain V-I region BAN | ⇑ 1.570 | 3.56E-04 |
| P19823 | 4 | Inter-alpha-trypsin inhibitor heavy chain H2 | ⇑ 1.561 | 9.33E-05 |
| B4DTM7 | 6 | cDNA FLJ53006, highly similar to Vinculin | ⇑ 1.555 | 1.08E-04 |
| P0CG05 | 2 | Ig lambda-2 chain C regions | ⇑ 1.546 | 5.51E-06 |
| Q5JZH0 | 2 | Cathepsin A | ⇑ 1.544 | 4.04E-07 |
| F6TTL5 | 2 | Actin-related protein 2/3 complex subunit 4 | ⇑ 1.521 | 4.44E-16 |
| Q86Y46 | 2 | Keratin, type II cytoskeletal 73 | ⇓ 31.745 | 1.46E-10 |
| P13647 | 2 | Keratin, type II cytoskeletal 5 | ⇓ 18.968 | 2.87E-13 |
| C9JKY1 | 11 | Junction plakoglobin | ⇓ 10.649 | 3.68E-05 |
| O75556 | 4 | Mammaglobin-B | ⇓ 7.969 | 3.65E-05 |
| Q5T3N1 | 8 | Annexin A1 | ⇓ 7.875 | 1.85E-04 |
| P04792 | 8 | Heat shock protein beta-1 | ⇓ 6.669 | 2.12E-04 |
| O95171 | 7 | Sciellin | ⇓ 6.573 | 1.32E-12 |
| Q7Z3Z0 | 2 | Keratin, type I cytoskeletal 25 | ⇓ 6.165 | 1.99E-04 |
| P02545 | 7 | Lamin A/C | ⇓ 5.924 | 1.86E-04 |
| Q6ZN66 | 2 | Guanylate-binding protein 6 | ⇓ 5.722 | 7.44E-08 |
| Q96FQ6 | 3 | Protein S100-A16 | ⇓ 5.472 | 5.30E-11 |
| P22735 | 8 | Protein-glutamine gamma-glutamyltransferase K | ⇓ 4.374 | 9.85E-04 |
| P62851 | 3 | 40S ribosomal protein S25 | ⇓ 4.158 | 3.64E-10 |
| B4DJ43 | 3 | cDNA FLJ53341, highly similar to Tubulin beta-4 chain | ⇓ 3.325 | 1.11E-15 |
| P25705 | 3 | ATP synthase subunit alpha, mitochondrial | ⇓ 3.091 | 1.32E-06 |
| Q5SQY1 | 3 | Novel protein similar to beta-tubulin 4Q | ⇓ 3.070 | 3.09E-05 |
| P63241-2 | 3 | Isoform 2 of Eukaryotic translation initiation factor 5A-1 | ⇓ 3.034 | 9.12E-04 |
| P25398 | 4 | 40S ribosomal protein S12 | ⇓ 2.833 | 5.79E-06 |
| P68104 | 3 | Elongation factor 1-alpha 1 | ⇓ 2.801 | 1.06E-07 |
| P61026 | 3 | Ras-related protein Rab-10 | ⇓ 2.731 | 1.08E-05 |
| O60218 | 6 | Aldo-keto reductase family 1 member B10 | ⇓ 2.605 | 9.68E-06 |
| B4DPU3 | 4 | cDNA FLJ56548, highly similar to Elongation factor 2 | ⇓ 2.566 | 4.55E-12 |
| A8K2U0 | 11 | Alpha-2-macroglobulin-like protein 1 | ⇓ 2.522 | 3.61E-04 |
| Q9NZT1 | 7 | Calmodulin-like protein 5 | ⇓ 2.488 | 6.82E-04 |
| P07951 | 3 | Tropomyosin 2 (Beta) | ⇓ 2.314 | 7.67E-04 |
| P30838 | 2 | Aldehyde dehydrogenase, dimeric NADP-preferring | ⇓ 2.049 | 1.40E-10 |
| B4DLA9 | 2 | Histone H2B | ⇓ 2.018 | 1.64E-13 |
| P29373 | 2 | Cellular retinoic acid binding protein 2 | ⇓ 1.997 | 3.27E-09 |
| Q6LES2 | 4 | Annexin | ⇓ 1.984 | 4.06E-04 |
| Q5JR95 | 2 | Ribosomal protein S8 | ⇓ 1.978 | 2.93E-04 |
| ALDH3A1 | 4 | ldehyde dehydrogenase, dimeric NADP-preferring | ⇓ 1.940 | 1.36E-10 |
| P05120 | 3 | Plasminogen activator inhibitor 2 | ⇓ 1.894 | 2.05E-10 |
| Q9Y2V2 | 2 | Calcium-regulated heat stable protein 1 | ⇓ 1.792 | 1.36E-11 |
| P50990 | 3 | T-complex protein 1 subunit theta | ⇓ 1.729 | 1.91E-10 |
| P08493 | 2 | Matrix Gla protein | ⇓ 1.699 | 3.08E-08 |
| 075390 | 3 | Citrate synthase, mitochondrial | ⇓ 1.516 | 2.06E-07 |
⇑ Represents upregulation and ⇓ Represents downregulation
Table 4.
Differentially expressed salivary proteins in low BP infusion BRONJ group compared to high BP infusion control group
| Accession Number | Unique Peptides | Protein Name | Fold Change† | P value |
|---|---|---|---|---|
| Low BP Infusion BRONJ Group (i116) vs High BP Infusion Control Group (i115) | ||||
| Q86Y46 | 2 | Keratin, type II cytoskeletal 73 | ⇓ 6.554 | 2.11E-15 |
| P02538 | 28 | Keratin, type II cytoskeletal 6A | ⇓ 6.022 | 1.02E-07 |
| P13646 | 50 | Keratin, type I cytoskeletal 13 | ⇓ 5.558 | 6.80E-07 |
| P12035 | 10 | Keratin, type II cytoskeletal 3 | ⇓ 5.302 | 1.20E-07 |
| B4DRW1 | 40 | DNA FLJ55805, highly similar to Keratin, type II cytoskeletal 4 | ⇓ 5.222 | 3.23E-05 |
| P14923 | 11 | Junction plakoglobin | ⇓ 5.001 | 3.44E-15 |
| P08727 | 8 | Keratin, type I cytoskeletal 19 | ⇓ 4.861 | 2.94E-05 |
| Q01546 | 11 | Keratin, type II cytoskeletal 2 oral | ⇓ 4.850 | 1.60E-06 |
| P08779 | 40 | Keratin, type I cytoskeletal 16 | ⇓ 4.632 | 8.83E-05 |
| P47929 | 6 | Galectin-7 | ⇓ 4.624 | 8.57E-06 |
| P19012 | 2 | Keratin, type I cytoskeletal 15 | ⇓ 4.453 | 1.55E-15 |
| Q5T3N1 | 8 | Annexin | ⇓ 4.438 | 3.56E-07 |
| P07355 | 8 | Annexin A2 | ⇓ 4.307 | 7.90E-08 |
| P13647 | 11 | Keratin, type II cytoskeletal 5 | ⇓ 4.090 | 4.62E-04 |
| Q9HCY8 | 6 | Protein S100-A14 | ⇓ 4.050 | 7.72E-07 |
| Q8N1N4 | 14 | Keratin, type II cytoskeletal 78 | ⇓ 4.035 | 3.74E-05 |
| P15924 | 43 | Desmoplakin | ⇓ 3.942 | 1.38E-04 |
| P02533 | 10 | Keratin, type I cytoskeletal 14 | ⇓ 3.803 | 9.50E-04 |
| Q7Z3Z0 | 2 | Keratin, type I cytoskeletal 25 | ⇓ 3.782 | 2.11E-15 |
| Q13835 | 12 | Plakophilin-1 | ⇓ 3.751 | 1.59E-05 |
| P04083 | 14 | Annexin A1 | ⇓ 3.544 | 2.76E-04 |
| P04792 | 8 | Heat shock protein beta-1 | ⇓ 3.477 | 2.77E-11 |
| P20930 | 2 | Filaggrin | ⇓ 3.431 | 3.03E-14 |
| B4DLA9 | 2 | Histone H2B type 2-F | ⇓ 3.332 | 2.89E-15 |
| P22735 | 8 | Protein-glutamine gamma-glutamyltransferase K | ⇓ 3.330 | 1.99E-12 |
| O60437 | 39 | Periplakin | ⇓ 3.283 | 3.70E-05 |
| Q92817 | 23 | Envoplakin | ⇓ 3.129 | 3.82E-06 |
| Q6ZN66 | 9 | Guanylate-binding protein 6 | ⇓ 3.061 | 2.48E-07 |
| P62851 | 3 | 40S ribosomal protein S25 | ⇓ 3.041 | 3.99E-14 |
| Q9UBG3 | 9 | Cornulin | ⇓ 3.029 | 7.81E-05 |
| P19013 | 3 | Keratin, type II cytoskeletal 4 | ⇓ 2.821 | 7.85E-04 |
| B4DQ53 | 6 | cDNA FLJ51275 | ⇓ 2.690 | 2.16E-06 |
| B4DPU3 | 4 | cDNA FLJ56548, highly similar to Elongation factor 2 | ⇓ 2.642 | 6.11E-15 |
| P05388 | 3 | 60S acidic ribosomal protein P0 | ⇓ 2.618 | 5.69E-06 |
| P63241 | 3 | Eukaryotic translation initiation factor 5A-1 | ⇓ 2.605 | 3.89E-15 |
| Q96QV6 | 3 | Histone H2A type 1-A | ⇓ 2.580 | 5.75E-12 |
| Q9H4B7 | 4 | Tubulin beta-1 chain | ⇓ 2.553 | 3.97E-04 |
| Q16778 | 9 | Histone H2B type 2-E | ⇓ 2.543 | 8.52E-06 |
| Q5JR95 | 2 | 40S ribosomal protein S8 | ⇓ 2.530 | 1.52E-06 |
| Q5SQY1 | 3 | Novel protein similar to beta-tubulin 4Q | ⇓ 2.472 | 8.29E-14 |
| O75367 | 3 | Core histone macro-H2A.1 | ⇓ 2.405 | 9.82E-08 |
| Q59GP5 | 3 | Eukaryotic translation elongation factor 1 alpha 2 variant | ⇓ 2.405 | 6.65E-05 |
| P01040 | 8 | Cystatin-A | ⇓ 2.321 | 1.97E-05 |
| P08238 | 17 | Heat shock protein HSP 90-beta | ⇓ 2.250 | 6.54E-10 |
| P31947 | 18 | Epithelial cell marker protein 1 | ⇓ 2.240 | 6.99E-15 |
| P16401 | 4 | Histone H1.5 | ⇓ 2.193 | 4.39E-08 |
| Q16695 | 3 | Histone H3.1t | ⇓ 2.181 | 6.89E-08 |
| P15515 | 6 | Histatin-1 | ⇓ 2.158 | 5.53E-05 |
| P14555 | 3 | Phospholipase A2, membrane associated | ⇓ 2.134 | 1.43E-14 |
| P50990 | 3 | T-complex protein 1 subunit theta | ⇓ 2.132 | 1.55E-15 |
| O15335 | 2 | Chondroadherin | ⇓ 2.112 | 1.82E-14 |
| Q16610 | 5 | Extracellular matrix protein 1 | ⇓ 2.091 | 6.00E-10 |
| Q08188 | 26 | Protein-glutamine gamma-glutamyltransferase E | ⇓ 2.090 | 2.09E-04 |
| P07476 | 18 | Involucrin | ⇓ 2.057 | 2.55E-06 |
| P62805 | 10 | Histone H4 | ⇓ 2.050 | 2.96E-08 |
| P55072 | 10 | Transitional endoplasmic reticulum ATPase | ⇓ 2.035 | 3.25E-11 |
| P25398 | 4 | 40S ribosomal protein S12 | ⇓ 1.975 | 4.44E-16 |
| P13645 | 24 | Keratin, type I cytoskeletal 10 | ⇓ 1.974 | 3.20E-04 |
| P48643 | 3 | T-complex protein 1 subunit epsilon | ⇓ 1.913 | 6.05E-07 |
| P49189 | 3 | 4-trimethylaminobutyraldehyde dehydrogenase | ⇓ 1.897 | 3.71E-04 |
| Q15149 | 4 | Plectin | ⇓ 1.896 | 1.78E-15 |
| Q00610 | 7 | Clathrin heavy chain 1 | ⇓ 1.892 | 3.09E-05 |
| P07951 | 3 | Tropomyosin beta chain | ⇓ 1.886 | 6.40E-06 |
| P63104 | 10 | Protein kinase C inhibitor protein 1 | ⇓ 1.882 | 8.76E-10 |
| P07900 | 5 | Heat shock protein HSP 90-alpha | ⇓ 1.871 | 4.96E-06 |
| Q15366 | 2 | Poly (rC)-binding protein 2 | ⇓ 1.862 | 2.79E-09 |
| P40394 | 4 | Alcohol dehydrogenase class 4 mu/sigma chain | ⇓ 1.809 | 3.25E-04 |
| P30838 | 7 | Aldehyde dehydrogenase, dimeric NADP-preferring | ⇓ 1.808 | 7.64E-07 |
| P13489 | 5 | Ribonuclease inhibitor | ⇓ 1.803 | 9.43E-08 |
| P07384 | 2 | Calpain-1 catalytic subunit | ⇓ 1.791 | 4.00E-15 |
| P46940 | 19 | Ras GTPase-activating-like protein IQGAP1 | ⇓ 1.791 | 5.10E-06 |
| Q7Z406 | 4 | Myosin-14 | ⇓ 1.751 | 7.46E-14 |
| A8K2U0 | 11 | Alpha-2-macroglobulin-like protein 1 | ⇓ 1.749 | 1.23E-10 |
| P13797 | 6 | Plastin-3 | ⇓ 1.689 | 2.11E-05 |
| P35579 | 52 | Myosin-9 | ⇓ 1.689 | 7.61E-06 |
| B4DTG2 | 5 | Elongation factor 1-gamma | ⇓ 1.676 | 5.69E-07 |
| P17858 | 5 | 6-phosphofructokinase, liver type | ⇓ 1.667 | 5.46E-06 |
| P06576 | 11 | ATP synthase subunit beta, mitochondrial | ⇓ 1.667 | 2.78E-04 |
| Q15084 | 5 | Protein disulfide-isomerase A6 | ⇓ 1.654 | 2.76E-06 |
| P36952 | 6 | Serpin B5 | ⇓ 1.651 | 5.93E-07 |
| P07384 | 7 | Calpain-1 catalytic subunit | ⇓ 1.650 | 1.33E-05 |
| Q16851 | 6 | UTP--glucose-1-phosphate uridylyltransferase | ⇓ 1.634 | 2.35E-07 |
| P14625 | 3 | Endoplasmin | ⇓ 1.615 | 6.29E-04 |
| P09525 | 4 | Annexin A4 | ⇓ 1.606 | 2.22E-14 |
| P10809 | 3 | 60 kDa heat shock protein, mitochondrial | ⇓ 1.594 | 2.07E-11 |
| P60660 | 8 | Myosin light polypeptide 6 | ⇓ 1.585 | 2.84E-09 |
| Q9UHA7 | 7 | Interleukin-36 alpha | ⇓ 1.569 | 2.18E-04 |
| Q9Y2V2 | 2 | Calcium-regulated heat stable protein 1 | ⇓ 1.564 | 6.00E-15 |
| P13693 | 2 | Translationally-controlled tumor protein | ⇓ 1.526 | 1.66E-07 |
| Q7L7L0 | 4 | Histone H2A type 3 | ⇓ 1.519 | 4.72E-04 |
| Q06830 | 8 | Peroxiredoxin-1 | ⇓ 1.519 | 5.99E-04 |
| P62879 | 2 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-2 | ⇓ 1.517 | 2.98E-10 |
| P30041 | 10 | Peroxiredoxin-6 | ⇓ 1.503 | 1.13E-07 |
⇑ Represents upregulation and ⇓ Represents downregulation
Bioinformatics for Prioritizing and Selecting Biomarker Candidates
Since validating all the identified proteins is time-consuming and expensive, we sought to prioritize the 200 proteins that were significantly altered in the three comparisons made between cases and controls based on number of BP infusions. Towards this end, we used IPA software to group differentially expressed proteins into functional pathways. We postulated that such grouping would reveal proteins with possible ties to BRONJ development. The most significantly represented pathways were predicted to have a role in drug metabolism and molecular transport in comparison-I; cellular movement, hematological function and immune cell trafficking in comparison-II; and dermatological diseases, developmental disorder and organismal abnormalities in comparison-III. Among the prioritized proteins, we selected matrix metalloproteinase-9 (MMP-9) and desmoplakin as candidates for further validation. Our previous study indicated that MMP-9 is up-regulated in tooth extraction sockets of BP treated rats (Basi et al. 2011). Also, it has been shown that BP treatment has cytotoxic effects on the oral mucosa and delays epithelial wound healing in in vitro and in vivo models (Landesberg et al. 2008, Mawardi et al. 2011). Therefore, we sought to validate desmoplakin, the most abundant desmosomal protein that is essential for maintaining the structural integrity of the epithelium.
Validation of Biomarker Candidates by Immunoassays
Enzyme-Linked Immunosorbent Assay (ELISA) was performed to detect the levels of MMP-9 (Quantikine, R&D Systems, Minneapolis, MN) and desmoplakin (Blue Gene, Life Sciences Advanced Technologies Inc, Saint Petersburg, FL) in individual saliva and serum samples of cases (n=20) and controls (n=20) according to the manufacturer's instructions. We analyzed serum samples because true biological changes in protein expression will also be manifested in other biofluids apart from saliva. Similar to 2D-LC-MS, immunoassays confirmed the increased expression of MMP-9 in saliva (mean±SD, 635±742 vs 280±227 ng/mL, p=0.048) and serum samples (426±386 vs 246±161ng/mL, p=0.05) of BRONJ subjects compared to controls. Desmoplakin levels were decreased in BRONJ serum samples compared to controls, although it was not statistically significant (1.9±2.0 vs 2.8±2.0 ng/mL, p=0.157). Desmoplakin expression couldn't be detected in saliva samples; a possible explanation is that the primary antibody was not sensitive enough to detect desmoplakin expression in saliva. Nevertheless, desmoplakin serum result has a similar trend to that of salivary results obtained through 2D-LC-MS.
Despite our attempts to match cases and control for BP infusions and cancer type, BRONJ cases had significantly higher BP infusions than controls. To address this imbalance, we compared MMP-9 and desmoplakin levels from the ELISA assays between a small subgroup of BRONJ cases (n=7) and controls (n=7) who we closely matched for mean age (±2 years), sex (female), cancer type (i.e., all had breast cancer), and number of BP infusions (± 4 infusions). Within this well-matched subgroup, MMP-9 levels remained significantly higher in BRONJ cases compared to controls (460±349 vs 154±112ng/mL, p=0.05). Serum MMP-9 levels also were higher in BRONJ cases compared to controls (240±221 vs 203±135 ng/mL), although the difference was not significant (p=0.72). Again consistent with the results seen in the entire cohort, serum desmoplakin levels among the well-matched individuals were significantly lower in BRONJ cases compared to controls (1.8±1.7 vs 4.2±2.1 ng/mL, p=0.03).
Discussion
To date, no validated biomarker exists for BRONJ. With the increasing use of BP's by millions of patients worldwide, there is a critical need to identify at “risk patients” before they develop clinical manifestations of BRONJ. To our knowledge, this is the first study to identify differentially expressed salivary proteins in BRONJ patients that could serve as potential biomarkers for BRONJ diagnosis. Using a comprehensive and rigorous proteomic analysis, we identified 200 proteins that were significantly differentially expressed in BRONJ cases compared to controls. Many of the identified proteins require further scrutiny. As a preliminary step, we sought to examine MMP-9 and desmoplakin in the present study and found MMP-9 to be increased and desmoplakin to be decreased in BRONJ cases compared to controls.
Matrix metalloproteinase-9 (MMP-9)
MMP-9 is a key proteolytic enzyme involved in wound healing (Colnot et al. 2003, Salo et al. 1994), and is essential for normal bone development and remodeling (Vu et al. 1998). MMP-9 degrades collagen fibrils, basement membranes, and other supra-structures of the extracellular matrix (Murphy et al. 2002, Sternlicht and Werb 2001). It is primarily expressed by osteoclasts, but is also expressed by neutrophils, macrophages and fibroblasts during wound healing process (Angelov et al. 2004, Okada et al. 1995).
Several studies have evaluated the effects of BP on Matrix metalloproteinases (MMPs) expression in cancer cells (Giraudo et al. 2004, Heikkila et al. 2002). In premalignant and malignant conditions, BP's inhibit angiogenesis by targeting both macrophage expression and proteolytic activity of MMPs (Giraudo et al. 2004). However, the effects of BP on MMPs from the normal oral mucosal cells are unclear. Allam et al. noted a significant reduction in MMP-9 expression in oral epithelial cells following zoledronate treatment (Allam et al. 2011). In the present study, we noted increased MMP-9 expression in BRONJ cases. The reason for elevated MMP-9 expression in BRONJ cases is unclear.
Previously, higher levels of MMP-9 have been noted in fluids of chronic wounds such as venous ulcers, diabetic foot ulcers and non-healing burn wounds (Ladwig et al. 2002, Widgerow 2010, Wysocki et al. 1999). It is believed that persistent inflammatory cells such as neutrophils and macrophages in chronic wounds contribute to the steady elevated level of MMP-9, and these increased levels of MMP-9 have been associated with poor wound healing (Ladwig et al. 2002, Widgerow 2010, Wysocki et al. 1999). It is hypothesized that the prolonged, excessively proteolytic environment will continually degrade growth factors, receptors, and extracellular matrix proteins, thus affecting the ability to proceed into the normal remodeling stage of wound healing (Widgerow 2011). Some studies have even looked at decreasing the undesirable high levels of MMP-9 in chronic infected wounds, acute inflamed wounds and burn wounds to facilitate wound healing (Widgerow 2010). In the present study, it is possible that the increased expression of MMP-9 may not be the causative factor for BRONJ development per se but an observation related to persistent BRONJ infection. Cases had a history of BRONJ infection for an average period of 3±2 years. In addition, it is possible that the elevated level of MMP-9 in cases may have impacted the BRONJ healing process.
Furthermore, MMP-9 is significantly overexpressed in cancer cells and play an important role in breast cancer invasion, metastasis and tumor angiogenesis, and also affects cancer prognosis and survival rates (Djonov et al. 2002, Duffy et al. 2000, Jones et al. 1999). MMP-9 expression has been observed in neoplastic cell plasma membrane, non-neoplastic ducts and acini, stromal fibroblasts, endothelial cells, and tumor-infiltrating inflammatory cells including neutrophils, macrophages, and lymphocytes (Balduyck et al. 2000). In the present study, it is unknown if the increased expression of MMP-9 in cases is due to BP-mediated side effects (e.g., BRONJ) or due to the underlying biological phenomenon of existing cancer. Our previous clinical study that analyzed whole saliva from 10 healthy women and 10 metastatic breast cancer patients found no significant difference in MMP-9 expression between healthy individuals and breast cancer patients (Bandhakavi et al. 2011). Subjects in our previous study were free of confounding conditions that could alter salivary protein expression such as periodontal/autoimmune disease, history of other diseases or use of potentially interfering medications. None of the breast cancer patients were receiving BP therapy and to our knowledge had any evidence of BRONJ. Based on our previous findings, it can be hypothesized that any changes in MMP-9 expression in BRONJ subjects may have not been masked by the existing cancer or cancer progression in the present study.
Desmosomal proteins
Desmosomes are intercellular adhesive junctions that link epithelial cells to each other and attach keratin intermediate filament to the cell surface. This assembly not only allows epithelial tissues to withstand mechanical stress, but also facilitates cell-cell communication through signal transmission. Any disruption in the desmosome-keratin filament complex results in a breakdown in cell adhesion leading to various skin, hair, and heart defects (McGrath 2005).
Several genetic and autoimmune diseases involving desmosomal proteins such as desmoplakin have been described. Mutations that truncate the desmoplakin C-terminus results in a lethal condition termed acantholytic epidermolysis bullosa (Jonkman et al. 2005). Patients with skin fragility-ectodermal dysplasia syndrome have mutations in plakophilin-1 (McGrath et al. 1997) and exhibit severe acantholysis (loss of cell-cell adhesion), which highlights the importance of plakophilins in desmosomal adhesion. Autoantibodies to periplakin and envoplakin, members of the plakin family with structures similar to desmoplakin, are present in the mucocutaneous blistering-disease paraneoplastic pemphigus (Robinson et al. 1999). Furthermore, plakoglobin together with plakophilin-1 and 3, play important role in facilitating the adhesion of desmoplakin to keratin intermediate filaments and in mediating important signal transduction pathways (Hatzfeld 2007). Plakoglobin-knockout mice exhibit skin blistering from acantholysis, indicating that plakoglobins are essential in maintaining the structural integrity of the epidermis (Bierkamp et al. 1996).
Collectively, these mutations provide insight into the functional domains of desmoplakin proteins and their relevance to skin biology. In the present study, 2D-LC-MS analysis revealed desmoplakin, periplakin, envoplakin, junction plakoglobin, and plakophilin-1 expressions were significantly downregulated in BRONJ cases compared to controls (Table 4). Immunoassay performed on individual serum samples confirmed decreased expression of desmoplakin in BRONJ subjects. It is unclear if BP therapy affects desmosomal proteins, or if patients with desmosomal protein insufficiency are at a higher risk of developing BRONJ. Previously published reports have noted that 40-50% of the BRONJ lesions occur spontaneously without an obvious precipitating factor (Marx et al. 2007, Thumbigere-Math et al. 2012). It is possible that impairment in desmosomal proteins could lead to a compromise in the structural integrity of the epithelium leading to spontaneous BRONJ development.
Recently, several studies have suggested that the loss of desmosomal proteins is an early step in carcinogenesis (Beaudry et al. 2010, Chun and Hanahan 2010). Desmosomal proteins act as a tumor suppressor molecules and its decreased level is associated with invasive behavior in breast cancer, lung cancer, oropharyngeal squamous cell carcinoma, and pancreatic cancer (Hamidov et al. 2011, Oshiro et al. 2005, Papagerakis et al. 2009, Yang et al. 2012). In the present study, it is unknown if the decreased expression of desmoplakin in cases is due to BP-mediated side effects (e.g., BRONJ) or due to underlying cancer. Our previous study as well as other studies have not indicated any significant differences in salivary desmoplakin levels in cancer patients compared to healthy individuals (Bandhakavi et al. 2011, Streckfus et al. 2008, Zhang et al. 2010). Therefore, it is reasonable to assume that the decreased expression of desmoplakin in cases in the present study is related to BRONJ and not due to underlying cancer or cancer progression.
Furthermore, using proteomics approaches, we found keratins to be significantly downregulated in BRONJ cases compared to controls. Previous studies have demonstrated that BP's suppress cellular proliferation and delay wound healing of oral keratinocytes (Landesberg et al. 2008). Mawardi et al., in a murine model, showed that the combination of pamidronate and Fusobacterium nucleatum caused BRONJ-like lesions and delayed epithelial wound healing as result of diminished production of keratinocyte growth factor by gingival fibroblasts (Mawardi et al. 2011). In an in vitro model, Kim et al. demonstrated that pamidronate induced senescence in normal human oral keratinocytes and impaired re-epithelialization of oral mucosa (Kim et al. 2011). Altogether, these findings suggest that impairment in cell-junction–related proteins may play a vital role in BRONJ development and affect BRONJ healing process. It is possible that higher concentrations of BP's in jawbones may cause direct toxicity to the oral epithelium affecting cell-junction–related proteins.
This cross-sectional study has several limitations. Despite attempts to match cases and controls according to BP exposure, the number of BP infusions differed significantly between BRONJ cases and controls. The disparity in BP treatment, cancer type and gender distribution between the groups could explain some of the differences in protein expression between BRONJ subjects and controls. Notably, when limited number of cases and controls were matched according to age, sex, cancer, and number of BP infusions, MMP-9 expression in saliva and desmoplakin expression in serum showed statistically significant differences similar to 2D-LC-MS results. Also, the discontinuation of BP therapy in BRONJ cases prior to the study enrolment may have affected our results. As a standard practice, patients are generally advised to discontinue BP treatment as soon as they are diagnosed with BRONJ, thus it is difficult to enroll BRONJ patients who continue to receive BP therapy. Nevertheless, results from this preliminary study provide insight into the pathophysiology of BRONJ development and raise several hypotheses for future research. One or more of the differentially expressed proteins identified by this study may prove to be useful biomarkers for BRONJ diagnosis. The role of MMP-9 and desmoplakin in BRONJ development requires further investigation.
Acknowledgements
We thank all the study participants for their commitment and support towards this project. This study was supported by a grant (R21 DE018717 to Dr. Gopalakrishnan) from the National Institute of Dental and Craniofacial Research. We thank the Center for Mass Spectrometry and Proteomics at the University of Minnesota for instrumental resources and the Minnesota Supercomputing Institute for computational support. We thank Dr. Ma'Ann Sabino and Carol Dunn for helping with patient recruitment and sample collection. We also thank Dr. Kim Mansky and Dr. Eric Jensen for their help with lab analysis.
References
- 1.Allam E, Allen M, Chu TM, Ghoneima A, Jack Windsor L. In vivo effects of zoledronic acid on oral mucosal epithelial cells. Oral diseases. 2011;17:291–7. doi: 10.1111/j.1601-0825.2010.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelov N, Moutsopoulos N, Jeong MJ, Nares S, Ashcroft G, Wahl SM. Aberrant mucosal wound repair in the absence of secretory leukocyte protease inhibitor. Thrombosis and haemostasis. 2004;92:288–97. doi: 10.1160/TH03-07-0446. [DOI] [PubMed] [Google Scholar]
- 3.Balduyck M, Zerimech F, Gouyer V, Lemaire R, Hemon B, Grard G, Thiebaut C, Lemaire V, Dacquembronne E, Duhem T, Lebrun A, Dejonghe MJ, Huet G. Specific expression of matrix metalloproteinases 1, 3, 9 and 13 associated with invasiveness of breast cancer cells in vitro. Clinical & experimental metastasis. 2000;18:171–8. doi: 10.1023/a:1006762425323. [DOI] [PubMed] [Google Scholar]
- 4.Bandhakavi S, Van Riper SK, Tawfik PN, Stone MD, Haddad T, Rhodus NL, Carlis JV, Griffin TJ. Hexapeptide libraries for enhanced protein PTM identification and relative abundance profiling in whole human saliva. Journal of proteome research. 2011;10:1052–61. doi: 10.1021/pr100857t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basi DL, Hughes PJ, Thumbigere-Math V, Sabino M, Mariash A, Lunos SA, Jensen E, Gopalakrishnan R. Matrix metalloproteinase-9 expression in alveolar extraction sockets of zoledronic Acid-treated rats. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2011;69:2698–707. doi: 10.1016/j.joms.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 6.Beaudry VG, Jiang D, Dusek RL, Park EJ, Knezevich S, Ridd K, Vogel H, Bastian BC, Attardi LD. Loss of the p53/p63 regulated desmosomal protein Perp promotes tumorigenesis. PLoS genetics. 2010;6:e1001168. doi: 10.1371/journal.pgen.1001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedogni A, Blandamura S, Lokmic Z, Palumbo C, Ragazzo M, Ferrari F, Tregnaghi A, Pietrogrande F, Procopio O, Saia G, Ferretti M, Bedogni G, Chiarini L, Ferronato G, Ninfo V, Lo Russo L, Lo Muzio L, Nocini PF. Bisphosphonate-associated jawbone osteonecrosis: a correlation between imaging techniques and histopathology. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2008;105:358–64. doi: 10.1016/j.tripleo.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 8.Bierkamp C, McLaughlin KJ, Schwarz H, Huber O, Kemler R. Embryonic heart and skin defects in mice lacking plakoglobin. Developmental biology. 1996;180:780–5. doi: 10.1006/dbio.1996.0346. [DOI] [PubMed] [Google Scholar]
- 9.Chun MG, Hanahan D. Genetic deletion of the desmosomal component desmoplakin promotes tumor microinvasion in a mouse model of pancreatic neuroendocrine carcinogenesis. PLoS genetics. 2010:6. doi: 10.1371/journal.pgen.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–33. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djonov V, Cresto N, Aebersold DM, Burri PH, Altermatt HJ, Hristic M, Berclaz G, Ziemiecki A, Andres AC. Tumor cell specific expression of MMP-2 correlates with tumor vascularisation in breast cancer. International journal of oncology. 2002;21:25–30. [PubMed] [Google Scholar]
- 12.Duffy MJ, Maguire TM, Hill A, McDermott E, O'Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast cancer research : BCR. 2000;2:252–7. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. The Journal of clinical investigation. 2004;114:623–33. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamidov Z, Altendorf-Hofmann A, Chen Y, Settmacher U, Petersen I, Knosel T. Reduced expression of desmocollin 2 is an independent prognostic biomarker for shorter patients survival in pancreatic ductal adenocarcinoma. Journal of clinical pathology. 2011;64:990–4. doi: 10.1136/jclinpath-2011-200099. [DOI] [PubMed] [Google Scholar]
- 15.Hatzfeld M. Plakophilins: Multifunctional proteins or just regulators of desmosomal adhesion? Biochimica et biophysica acta. 2007;1773:69–77. doi: 10.1016/j.bbamcr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Heikkila P, Teronen O, Moilanen M, Konttinen YT, Hanemaaijer R, Laitinen M, Maisi P, van der Pluijm G, Bartlett JD, Salo T, Sorsa T. Bisphosphonates inhibit stromelysin-1 (MMP-3), matrix metalloelastase (MMP-12), collagenase-3 (MMP-13) and enamelysin (MMP-20), but not urokinase-type plasminogen activator, and diminish invasion and migration of human malignant and endothelial cell lines. Anti-cancer drugs. 2002;13:245–54. doi: 10.1097/00001813-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, Migliorati CA, Ristic H. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. Journal of the American Dental Association. 2011;142:1243–51. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- 18.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, Cui J, Adamus A, Gagel RF, Hortobagyi GN. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–36. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones JL, Glynn P, Walker RA. Expression of MMP-2 and MMP-9, their inhibitors, and the activator MT1-MMP in primary breast carcinomas. The Journal of pathology. 1999;189:161–8. doi: 10.1002/(SICI)1096-9896(199910)189:2<161::AID-PATH406>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Jonkman MF, Pasmooij AM, Pasmans SG, van den Berg MP, Ter Horst HJ, Timmer A, Pas HH. Loss of desmoplakin tail causes lethal acantholytic epidermolysis bullosa. American journal of human genetics. 2005;77:653–60. doi: 10.1086/496901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim RH, Lee RS, Williams D, Bae S, Woo J, Lieberman M, Oh JE, Dong Q, Shin KH, Kang MK, Park NH. Bisphosphonates induce senescence in normal human oral keratinocytes. J Dent Res. 2011;90:810–6. doi: 10.1177/0022034511402995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10:26–37. doi: 10.1046/j.1524-475x.2002.10903.x. [DOI] [PubMed] [Google Scholar]
- 23.Landesberg R, Cozin M, Cremers S, Woo V, Kousteni S, Sinha S, Garrett-Sinha L, Raghavan S. Inhibition of oral mucosal cell wound healing by bisphosphonates. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2008;66:839–47. doi: 10.1016/j.joms.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarovici TS, Mesilaty-Gross S, Vered I, Pariente C, Kanety H, Givol N, Yahalom R, Taicher S, Yarom N. Serologic bone markers for predicting development of osteonecrosis of the jaw in patients receiving bisphosphonates. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2010;68:2241–7. doi: 10.1016/j.joms.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 25.Lehrer S, Montazem A, Ramanathan L, Pessin-Minsley M, Pfail J, Stock RG, Kogan R. Bisphosphonate-induced osteonecrosis of the jaws, bone markers, and a hypothesized candidate gene. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2009;67:159–61. doi: 10.1016/j.joms.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Marx RE, Cillo JE, Jr., Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2007;65:2397–410. doi: 10.1016/j.joms.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Mawardi H, Giro G, Kajiya M, Ohta K, Almazrooa S, Alshwaimi E, Woo SB, Nishimura I, Kawai T. A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. J Dent Res. 2011;90:1339–45. doi: 10.1177/0022034511420430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGrath JA. Inherited disorders of desmosomes. The Australasian journal of dermatology. 2005;46:221–9. doi: 10.1111/j.1440-0960.2005.00188.x. [DOI] [PubMed] [Google Scholar]
- 29.McGrath JA, McMillan JR, Shemanko CS, Runswick SK, Leigh IM, Lane EB, Garrod DR, Eady RA. Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nature genetics. 1997;17:240–4. doi: 10.1038/ng1097-240. [DOI] [PubMed] [Google Scholar]
- 30.Murphy G, Knauper V, Atkinson S, Butler G, English W, Hutton M, Stracke J, Clark I. Matrix metalloproteinases in arthritic disease. Arthritis research. 2002;3(4 Suppl):S39–49. doi: 10.1186/ar572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada Y, Naka K, Kawamura K, Matsumoto T, Nakanishi I, Fujimoto N, Sato H, Seiki M. Localization of matrix metalloproteinase 9 (92-kilodalton gelatinase/type IV collagenase = gelatinase B) in osteoclasts: implications for bone resorption. Laboratory investigation; a journal of technical methods and pathology. 1995;72:311–22. [PubMed] [Google Scholar]
- 32.Olsen JV, Schwartz JC, Griep-Raming J, Nielsen ML, Damoc E, Denisov E, Lange O, Remes P, Taylor D, Splendore M, Wouters ER, Senko M, Makarov A, Mann M, Horning S. A dual pressure linear ion trap Orbitrap instrument with very high sequencing speed. Molecular & cellular proteomics : MCP. 2009;8:2759–69. doi: 10.1074/mcp.M900375-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onsongo G, Stone MD, Van Riper SK, Chilton J, Wu B, Higgins L, Lund TC, Carlis JV, Griffin TJ. LTQ-iQuant: A freely available software pipeline for automated and accurate protein quantification of isobaric tagged peptide data from LTQ instruments. Proteomics. 2010;10:3533–8. doi: 10.1002/pmic.201000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oshiro MM, Kim CJ, Wozniak RJ, Junk DJ, Munoz-Rodriguez JL, Burr JA, Fitzgerald M, Pawar SC, Cress AE, Domann FE, Futscher BW. Epigenetic silencing of DSC3 is a common event in human breast cancer. Breast cancer research : BCR. 2005;7:R669–80. doi: 10.1186/bcr1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papagerakis S, Shabana AH, Pollock BH, Papagerakis P, Depondt J, Berdal A. Altered desmoplakin expression at transcriptional and protein levels provides prognostic information in human oropharyngeal cancer. Human pathology. 2009;40:1320–9. doi: 10.1016/j.humpath.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Robinson ND, Hashimoto T, Amagai M, Chan LS. The new pemphigus variants. Journal of the American Academy of Dermatology. 1999;40:649–71. 672–3. doi: 10.1016/s0190-9622(99)70145-3. quiz. [DOI] [PubMed] [Google Scholar]
- 37.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Molecular & cellular proteomics : MCP. 2004;3:1154–69. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2009;67:2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. Journal of Oral and Maxillofacial Surgery. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Salo T, Makela M, Kylmaniemi M, Autio-Harmainen H, Larjava H. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Laboratory investigation; a journal of technical methods and pathology. 1994;70:176–82. [PubMed] [Google Scholar]
- 41.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annual review of cell and developmental biology. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Streckfus CF, Mayorga-Wark O, Arreola D, Edwards C, Bigler L, Dubinsky WP. Breast cancer related proteins are present in saliva and are modulated secondary to ductal carcinoma in situ of the breast. Cancer investigation. 2008;26:159–67. doi: 10.1080/07357900701783883. [DOI] [PubMed] [Google Scholar]
- 43.Thumbigere-Math V, Tu L, Huckabay S, Dudek AZ, Lunos S, Basi DL, Hughes PJ, Leach JW, Swenson KK, Gopalakrishnan R. A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. American journal of clinical oncology. 2012;35:386–92. doi: 10.1097/COC.0b013e3182155fcb. [DOI] [PubMed] [Google Scholar]
- 44.Treister NS, Friedland B, Woo SB. Use of cone-beam computerized tomography for evaluation of bisphosphonate-associated osteonecrosis of the jaws. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2010;109:753–64. doi: 10.1016/j.tripleo.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Vincenzi B, Napolitano A, Zoccoli A, Iuliani M, Pantano F, Papapietro N, Denaro V, Santini D, Tonini G. Serum VEGF levels as predictive marker of bisphosphonate-related osteonecrosis of the jaw. Journal of hematology & oncology. 2012;5:56. doi: 10.1186/1756-8722-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–22. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Widgerow AD. Nanocrystalline silver, gelatinases and the clinical implications. Burns. 2010;36:965–74. doi: 10.1016/j.burns.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Widgerow AD. Chronic wound fluid--thinking outside the box. Wound Repair Regen. 2011;19:287–91. doi: 10.1111/j.1524-475X.2011.00683.x. [DOI] [PubMed] [Google Scholar]
- 49.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nature methods. 2009;6:359–62. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 50.Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase-B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen. 1999;7:154–65. doi: 10.1046/j.1524-475x.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- 51.Xie H, Onsongo G, Popko J, de Jong EP, Cao J, Carlis JV, Griffin RJ, Rhodus NL, Griffin TJ. Proteomics analysis of cells in whole saliva from oral cancer patients via value-added three-dimensional peptide fractionation and tandem mass spectrometry. Molecular & cellular proteomics : MCP. 2008;7:486–98. doi: 10.1074/mcp.M700146-MCP200. [DOI] [PubMed] [Google Scholar]
- 52.Yang L, Chen Y, Cui T, Knosel T, Zhang Q, Albring KF, Huber O, Petersen I. Desmoplakin acts as a tumor suppressor by inhibition of the Wnt/beta-catenin signaling pathway in human lung cancer. Carcinogenesis. 2012;33:1863–70. doi: 10.1093/carcin/bgs226. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Xiao H, Karlan S, Zhou H, Gross J, Elashoff D, Akin D, Yan X, Chia D, Karlan B, Wong DT. Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PloS one. 2010;5:e15573. doi: 10.1371/journal.pone.0015573. [DOI] [PMC free article] [PubMed] [Google Scholar]