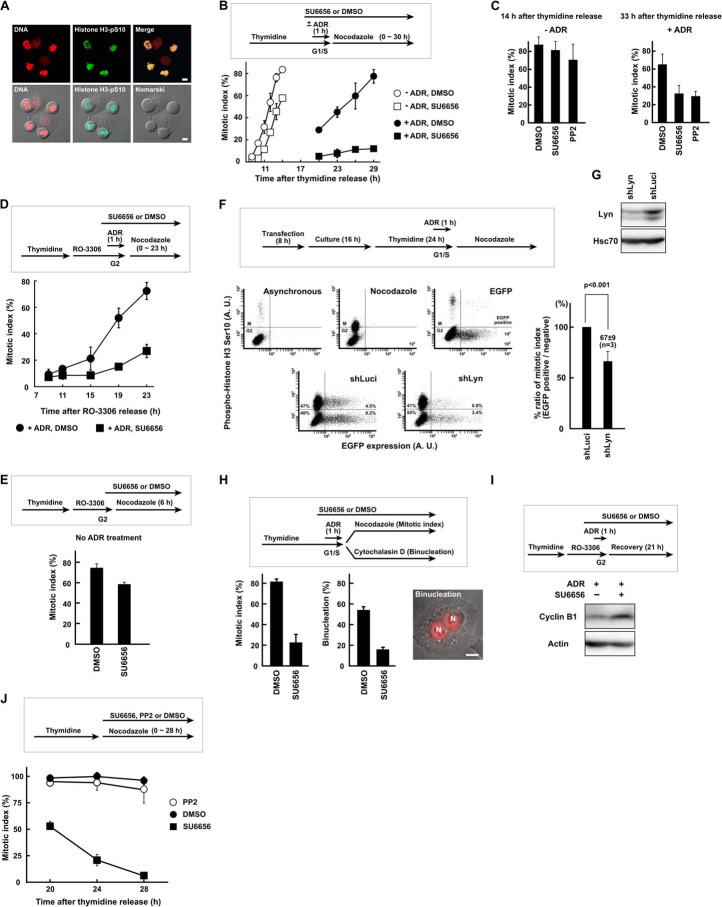
FIGURE 1.
SFK activity is required for G2 DNA damage checkpoint recovery. A, HeLa S3 cells with a round shape were stained with anti-phosphohistone H3 (Ser10) antibody and propidium iodide and confirmed as mitotic cells. B, HeLa S3 cells were synchronized at the G1/S phase boundary by thymidine block, exposed to 110 nm Adriamycin (ADR) for 1 h, and allowed to recover. Cells were exposed to 5 μm SU6656 or DMSO. Results without Adriamycin are also shown. C, HeLa S3 cells were treated as in B except that 20 μm PP2 was used. The mitotic index was determined at 14 and 33 h after release from thymidine synchronization for untreated (−ADR) and Adriamycin-treated (+ADR) cells, respectively. D and E, cells were treated as in B but synchronized in G2 phase with RO-3306. Results without Adriamycin are shown in E. F, HeLa S3 cells were transfected with shRNA expression vector targeting Lyn (shLyn). EGFP expression vector was co-transfected. Recovery from G2 checkpoint arrest was analyzed by flow cytometry as described under “Experimental Procedures.” An shRNA targeting luciferase (shLuci) was used as a control. p values were calculated using t test. A. U., arbitrary units. G, HeLa S3 cells were transfected with shRNA expression vector and cultured for 72 h. SDS lysates were prepared and probed with the indicated antibodies. H, Adriamycin-treated cells were cultured for 23 h in the presence of cytochalasin D to inhibit cytokinesis. The numbers of binucleated cells were counted, and the mitotic index was determined in parallel. N, nucleus. I, cells were exposed to Adriamycin as in D and harvested 21 h later. SDS lysates were prepared and probed for cyclin B1 and actin. J, HeLa S3 cells were synchronized at the G1/S phase boundary by thymidine block. Cells were released from thymidine and exposed to 5 μm SU6656, 20 μm PP2, or DMSO. The mitotic index was determined to monitor slippage from nocodazole-induced mitotic arrest. Scale bar, 10 μm for all panels. Error bars represent S.D.