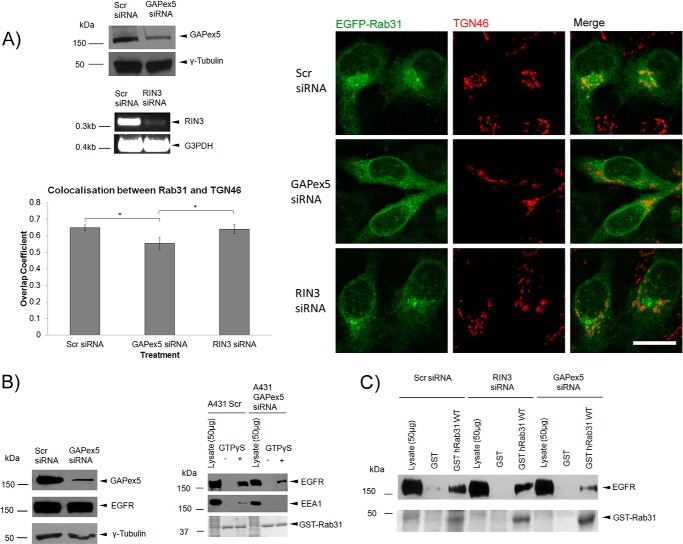
FIGURE 8.
The guanine nucleotide exchange factor GAPex5 is important for Rab31-EGFR interaction. A, A431 Rab31 OE cells were transfected with scrambled (Scr), GAPex5, or RIN3 siRNA and assayed after 48 h. Cell lysates were analyzed by Western blot analysis for GAPex5 knockdown and RT-PCR for RIN3 knockdown. Cells were fixed and immunostained for localization of EGFP-Rab31 (green) and TGN46 (red), a marker for the trans-Golgi network. Scale bar = 20 μm. Colocalization between EGFP-Rab31 and TGN46 was quantified using Zen 2010 software for calculation of the overlap coefficient. 27 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. *, p < 0.05; Student's t test. B, A431 cells were transfected with GAPex5 siRNA and harvested 48 h later. Left panel, the extent of GAPex5 knockdown and the levels of the EGFR were assessed by Western blot analysis. Right panel, a GST-Rab31 affinity pulldown assay was performed with the harvested lysates. Cells were pulsed with 0.5 μg/ml EGF before harvesting after a 30-min chase. 1 mg of lysates with and without GTPγS was incubated with 20 μg of GST-Rab31 and glutathione beads. The ability of GST-Rab31 to pull down EGFR and EEA1 was analyzed by Western blot analysis. Ponceau S staining of the GST proteins used is shown. C, 1 mg of A431 cell lysate harvested 48 h after transfection with relevant siRNA was incubated with 20 μg of GST or GST-Rab31 and glutathione beads in the presence of GTPγS, and the ability of the fusion proteins to pull down the EGFR was analyzed by Western blot analysis. GST proteins were visualized with Ponceau S stain.