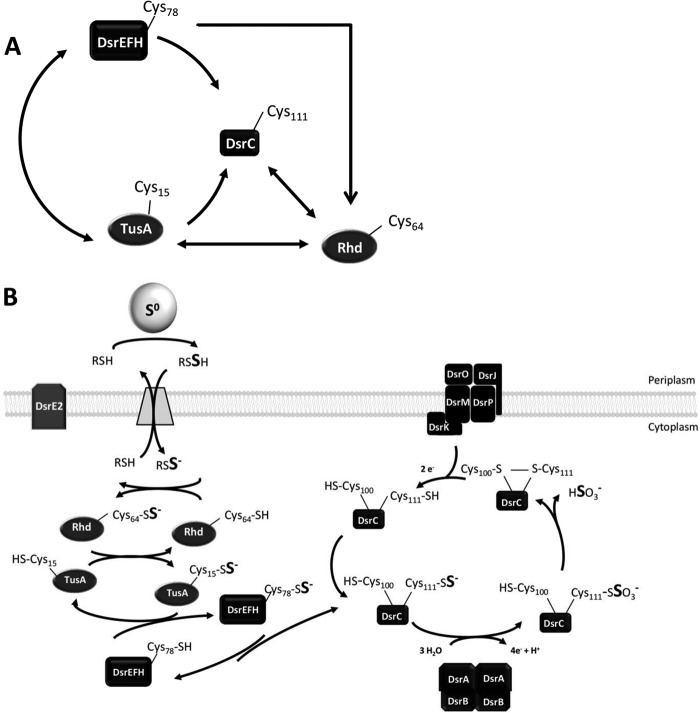
FIGURE 7.
Cytoplasmic sulfur trafficking during sulfur globule oxidation in A. vinosum. A, summary of sulfur transfer reactions detected in vitro. The cysteines responsible for sulfur binding are depicted. B, model of sulfur transfer reactions occurring in vivo. Rhd_2599 and TusA are introduced as sulfur-donating proteins for the Dsr system. Thiol groups and persulfides are shown in the ionized or protonated state according to their supposed pKa values of around 8.5 (46) and 6.2 (47), respectively. Because persulfides are 1–2 pKa units more acidic than their thiol equivalents, we calculated the pKa of the assumed carrier molecule glutathione amide persulfide on the basis of the pKa for glutathione (48) to be ∼7.2. DsrC-Cys-111 and Cys-100 correspond to CysA and CysB, respectively (9). See “Discussion” for detailed explanation.