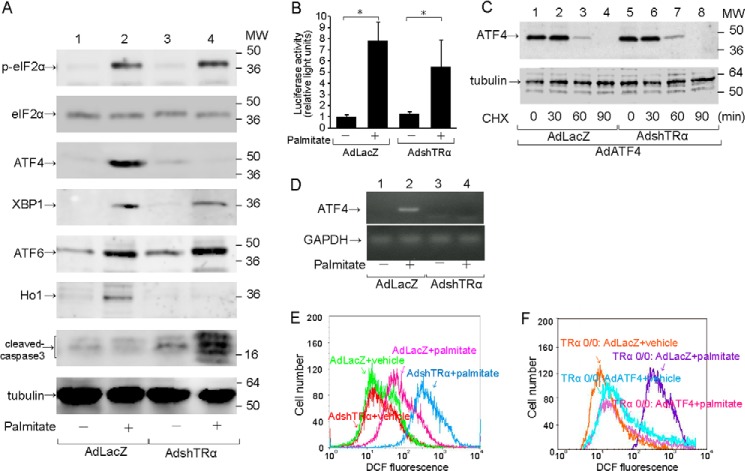
FIGURE 3.
Impaired eIF2α-ATF4 signaling pathway caused by deficiency of TRα. MIN6 cells were infected with 30 m.o.i. of AdLacZ or AdshTRα and incubated in medium containing 250 μm palmitate for an additional 24 h. A, to analyze the activation of the eIF2α-ATF4 signaling pathway, 20 μg of cell lysates prepared from adenovirus-infected MIN6 cells was immunoblotted with anti-total eIF2α antibody, anti-p-eIF2α antibody, anti-ATF4 antibody, anti-XBP1 antibody, anti-ATF6 antibody, anti-Ho1 antibody, and anti-cleaved-caspase3 antibody. Loading controls for tubulin are shown in the bottom panel. B, wild-type 5′-leader sequences of the ATF4 mRNA, which mediate translational control, were inserted between the constitutive thymidine kinase promoter and the firefly luciferase reporter gene. AdLacZ- or AdshTRα-infected MIN6 cells were co-transfected with the pTK-ATF4-Luc plasmid and a control Renilla luciferase plasmid. The transfected cells were treated with 250 μm palmitate. C, AdLacZ- or AdshTRα-infected MIN6 cells were co-infected with 30 m.o.i. of AdATF4 and treated with cycloheximide (CHX) (50 μg/ml) to block protein synthesis. At the indicated time points, cells were harvested, and 20 μg of extracts was analyzed for the protein levels of ATF4 by Western blotting. D, the expression of ATF4 and GAPDH mRNA was determined by real-time RT-PCR with 100 ng of cDNA. E and F, cellular ROS levels were measured by DCF fluorescence.