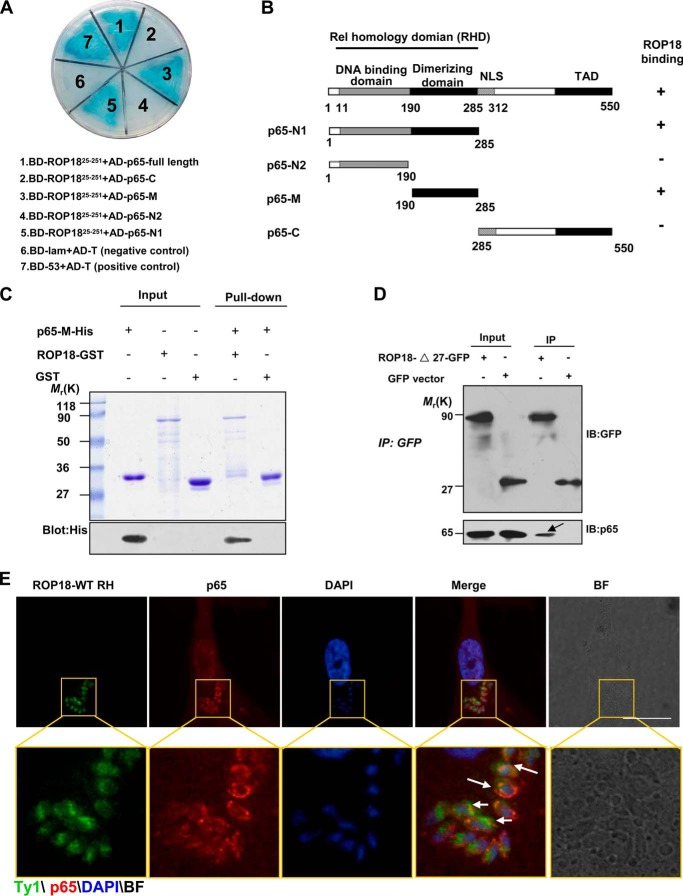
FIGURE 1.
Identification of p65 as a ROP18-interacting host protein. A, identification of the interaction of ROP18 with p65 using a yeast two-hybrid system. Screening plasmids expressing the N-terminal portion of ROP18 (amino acids 25–251, lacking signal peptide and kinase domain) fused to the GAL4 DNA binding domain were cotransfected with a plasmid expressing full-length or truncated versions of p65 fused to the GAL4 transactivation domain. Interactions were detected through growth on medium containing x-α-gal and lacking Ade, Trp, Leu, and His. Yeast cells co-transformed with BD-53 and AD-T were used as positive controls, and yeast cells co-transformed with BD-lam and AD-T were used as the negative controls. The results revealed that the dimerizing domain of p65 interacts with ROP18. B, schematic illustration of the full-length and truncated versions of p65. RHD, Rel homology domain (containing DNA binding domain and dimerizing domain); NLS, nuclear localization signal; TAD, transactivation domain. C, confirmation of the site of p65 binding to ROP18 using in vitro pulldown assays. GST-ROP18 purified on glutathione beads was used as an affinity matrix for absorbing His6-tagged p65-M fragments (dimerization domain of p65, amino acids 190–285). The SDS-PAGE gel was stained with Coomassie Brilliant Blue (upper panel) and subsequently blotted with anti-His antibody (lower panel). D, verifying the interaction of ROP18 with p65 through immunoprecipitation. 293T cells were transfected with ROP18-Δ27-GFP or control GFP vector. At 24 h after transfection, the cells were immunoprecipitated with anti-GFP. Starting fractions (Input) and immunoprecipitates (IP) were analyzed by SDS-PAGE and Western blotting using GFP and p65 antibodies. The arrow indicates that ROP18 (upper panel) and p65 (lower panel) were co-immunoprecipitated. E, HFF cells grown on glass coverslips were infected with or without ROP18-WT-Ty1 RH strain at an m.o.i. of 1 for 24 h. Double immunofluorescence was performed using mouse monoclonal anti-Ty1 (green) and rabbit polyclonal anti-p65 (red). A distinctive pattern of p65 and ROP18 localization was observed around the parasite. BF, brightfield. Bars, 5 μm.