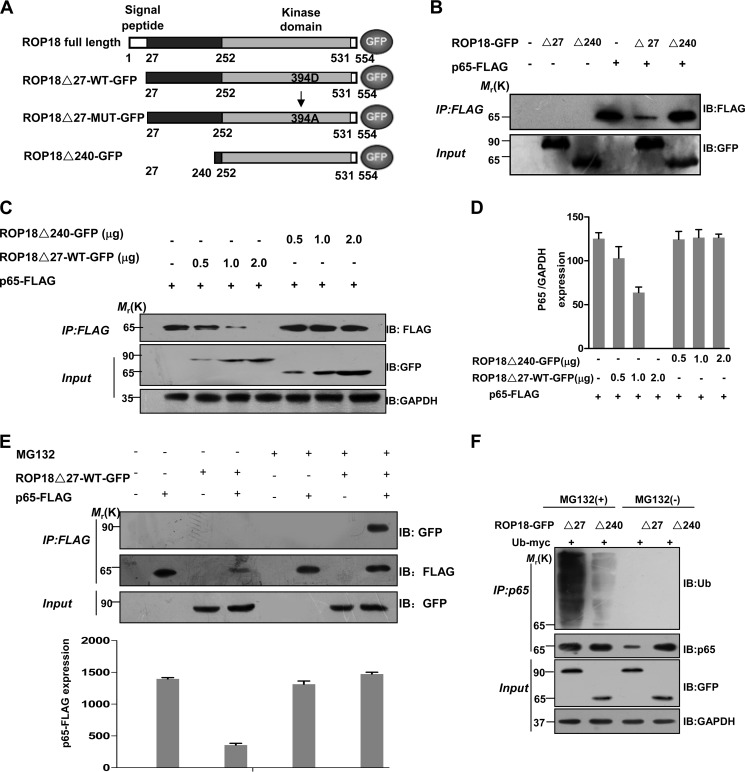
FIGURE 2.
ROP18 interacts with p65 and mediates its proteasome-ubiquitin-dependent degradation. A, schematic illustration of ROP18 and its mutant GFP-tagged expression vectors. ROP18Δ27-WT-GFP means lacking signal peptide (amino acids 1–27) ROP18 wild-type GFP-tagged plasmid; ROP18Δ27-MUT-GFP means lacking signal peptide kinase-deficient ROP18 GFP-tagged plasmid; ROP18Δ240-GFP means lacking N-terminal portion of ROP18 (amino acids 1–240). B, lysates of 293T cells transiently cotransfected with 2 μg of the indicated GFP-tagged ROP18 and/or 2 μg of FLAG-tagged p65 expression vectors were immunoprecipitated (IP) with the indicated antibodies and detected through Western blotting (IB) with the indicated antibodies. C, lysates of 293T cells transiently cotransfected with the indicated volumes of the GFP-tagged ROP18Δ27-WT or ROP18Δ240 and/or 2 μg of FLAG-tagged p65 expression vectors were immunoprecipitated with the indicated antibodies and detected through Western blotting with the indicated antibodies. D, the levels of p65 in the above experiment were quantified using the mean ratio of p65 to GAPDH for triplicate results. Error bars represent the means ± S.D. of triplicates. E, lysates of 293T cells transiently cotransfected with 2 μg of GFP-tagged ROP18Δ27-WT and/or 2 μg of FLAG-tagged p65 expression vectors in the absence or presence of MG132 (10 μm) for 12 h were immunoprecipitated with the indicated antibodies and detected through Western blotting. The levels of p65 in the above experiment were quantified for triplicate results. Error bars represent the means ± S.D. of triplicates. F, ROP18-p65 interaction increases p65 ubiquitination. 293T cells were transfected as indicated and cultured for 24 h, then the cells were treated with or without MG132 MG132 (10 μm) for 4 h before the cells were harvested. After immunoprecipitation of p65, its ubiquitination was shown by immunoblotting.