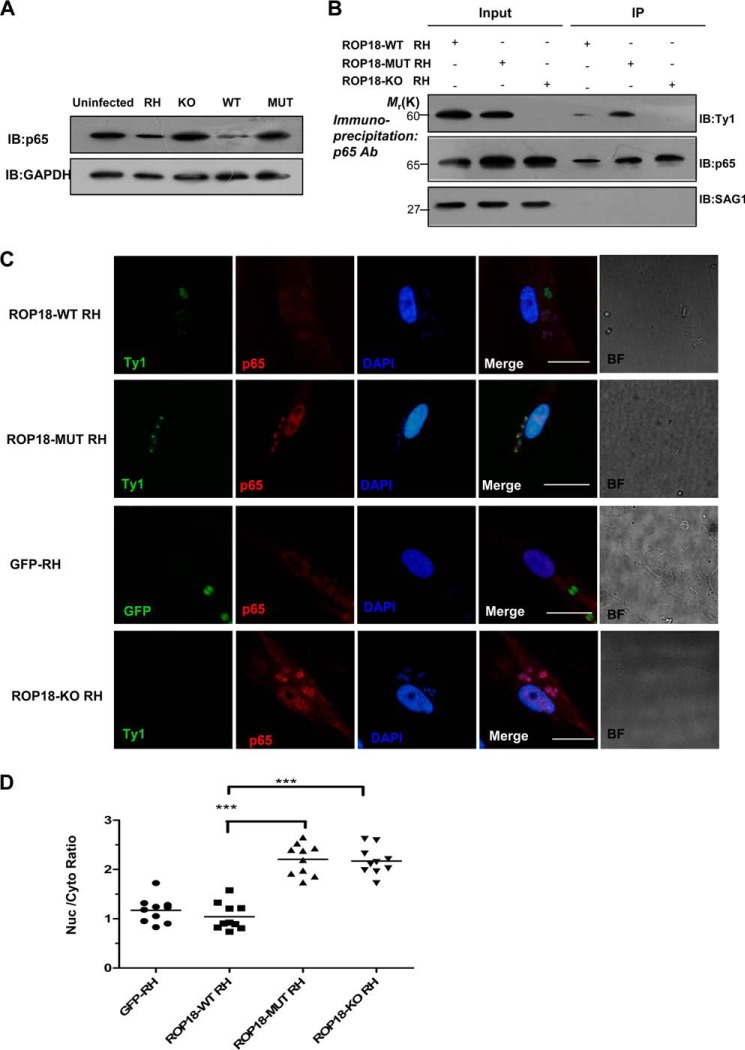
FIGURE 5.
Expressing wild-type ROP18 transgenic strains led to p65 degradation. A, the HFF cells were infected with the indicated parasites at an m.o.i. of 1. At 12 h after infection, the cells were lysed and subjected to Western blotting (IB) with anti-p65 (upper panel). The p65 expression levels were normalized against GAPDH (lower panel). B, kinase-deficient ROP18 forms a more stable interaction with p65 than wild-type ROP18 in infected cells. HFF cells were infected with the indicated strains for 12 h. Immunoprecipitation of p65 from infected cell lysates was detected with a polyclonal rabbit anti-p65. Input and immunoprecipitation (IP) fractions were resolved through SDS-PAGE and blotted for ROP18 using mouse monoclonal anti-Ty1. C, HFF cells grown on glass coverslips were infected with ROP18-WT RH strain, ROP18 MUT RH strain, ROP18-KO RH strain, or GFP-RH strain (stable expressing GFP RH strain) at an m.o.i. of 1 for >12 h. Double immunofluorescence was performed with mouse monoclonal anti-Ty1 (green) and rabbit polyclonal anti-p65 (red). A dramatic reduction of nuclear p65 was observed in ROP18-WT RH strain-infected cells compared with ROP18-MUT or ROP18-KO RH strain. BF, brightfield. Bars, 5 μm. D, the level of nuclear p65 in infected cells was quantified using the mean ratio of nuclear to cytoplasmic p65 (after subtraction of background signal) in at least 10 infected cells per stain. ***, p < 0.001.