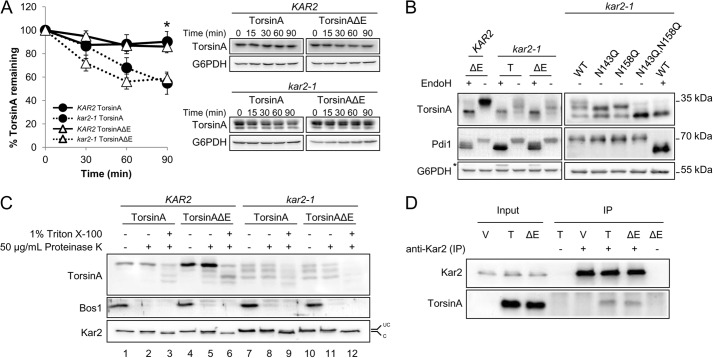
FIGURE 3.
TorsinA and torsinAΔE stability in yeast depends on the ER chaperone Kar2/BiP. A, log phase yeast cells expressing torsinA or torsinAΔE grown at 28 °C were treated with cycloheximide and then incubated at 37 °C for 90 min. Samples were taken every 30 min, and the amount of torsinA or torsinAΔE in each time point was quantified relative to that at the 0-min time point. Data represent the average ± S.E. of at least four experiments with ≥2 independent replicates/experiment. The % of torsinA or torsinAΔE remaining after CHX addition in wild-type KAR2 or kar2-1 yeast strains is graphed (*, p < 0.04 for KAR2 torsinA versus kar2-1 torsinA; p < 0.003 for KAR2 torsinAΔE versus kar2-1 torsinAΔE). Representative Western blots are shown. B, torsinA and torsinAΔE N-linked glycosylation is Kar2-dependent. Protein samples were prepared from log phase KAR2 and kar2-1 yeast strains as before, except that they were treated with endoglycosidase H (EndoH) as indicated before Western blot analysis. Pdi1 is an N-glycosylated protein, whereas G6PDH is a cytosolic unglycosylated protein. * indicates a background band in the blot. C, torsinA and torsinAΔE glycosylated species are ER-embedded. Microsomes from KAR2 and kar2-1 strains expressing torsinA and torsinAΔE were incubated on ice for 30 min in the presence or absence of Triton X-100, followed by a 1-h incubation on ice in the presence or absence of proteinase K. Proteins were TCA-precipitated, resolved by SDS-PAGE, and analyzed by Western blot. Note that the ER protein Kar2 is clipped by proteinase K in the presence of Triton X-100. Bos1 is an ER transmembrane protein with an epitope exposed to the cytosol that is proteinase K-sensitive even in the absence of Triton X-100. UC, uncleaved Kar2/BiP; C, cleaved Kar2/BiP. D, Kar2/BiP co-immunoprecipitates with torsinA and torsinAΔE. Whole cell extracts from KAR2 cells transformed with a vector control (V) or expression vectors for torsinA (T) or torsinAΔE (ΔE) were immunoprecipitated (IP) under native conditions (0.5% Triton X-100) using anti-Kar2 antiserum. Immunoprecipitated material was resolved by SDS-PAGE and analyzed by Western blot. Controls from immunoprecipitations in which primary antiserum was absent (−) were also included.