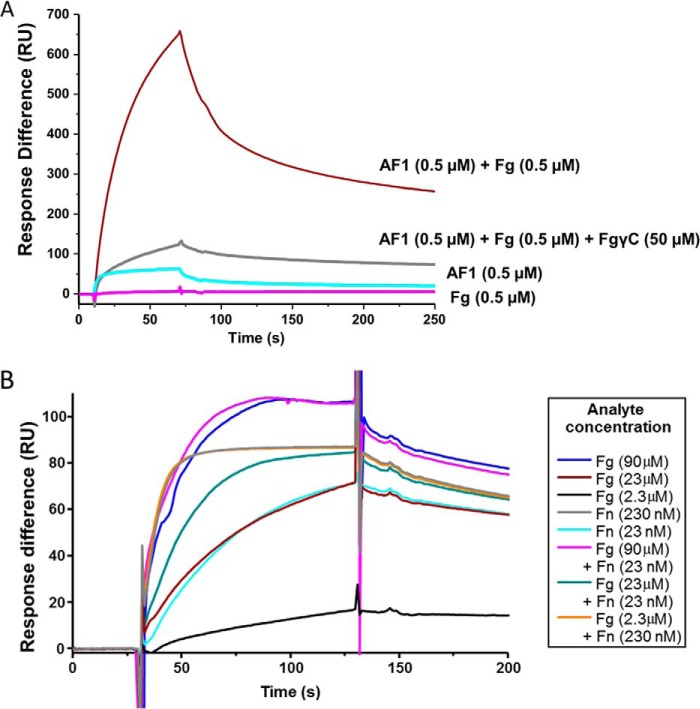
FIGURE 7.
Ternary complex formation and steric regulation. A, representative SPR sensorgrams show responses from the interactions of NTD (a proteolytic fragment of Fn) immobilized on a C1 chip with AF1 (0.5 μm; Fig. 1A) in the presence or absence of an equimolar concentration of Fg. The effect of FgγC (50 μm) on the NTD·AF1·Fg ternary complex is also shown. The experiments shown were conducted sequentially on the same chip, and the order of experiments was as follows: 0.5 μm AF1 + 0.5 μm Fg; 0.5 μm AF1 + 0.5 μm Fg + 50 μm FgγC; 0.5 μm Fg; and 0.5 μm AF1. Regeneration was achieved with low pH. Repeats of 0.5 μm AF1 + 0.5 μm Fg were performed midway and at the end of the sequence of experiments to check binding after regeneration (data not shown). B, representative SPR sensorgrams from interactions of AF1 (Fig. 1A) with Fn and/or Fg. The experiments shown were conducted sequentially on the same CM5 chip with immobilized AF1. The order of experiments was as follows: 90 μm Fg; 90 μm Fg + 23 nm Fn; 23 nm Fn; 23 μm Fg + 23 nm Fn; 23 μm Fg + 230 nm Fn; 23 μm Fg; 2.3 μm Fg; and 230 nm Fn. Regeneration was achieved with low pH. Repeats of 90 μm Fg and 23 nm Fn were performed well separated in the sequence of experiments to demonstrate reproducibility of Fg and Fn binding after regeneration (data not shown). RU, response units.