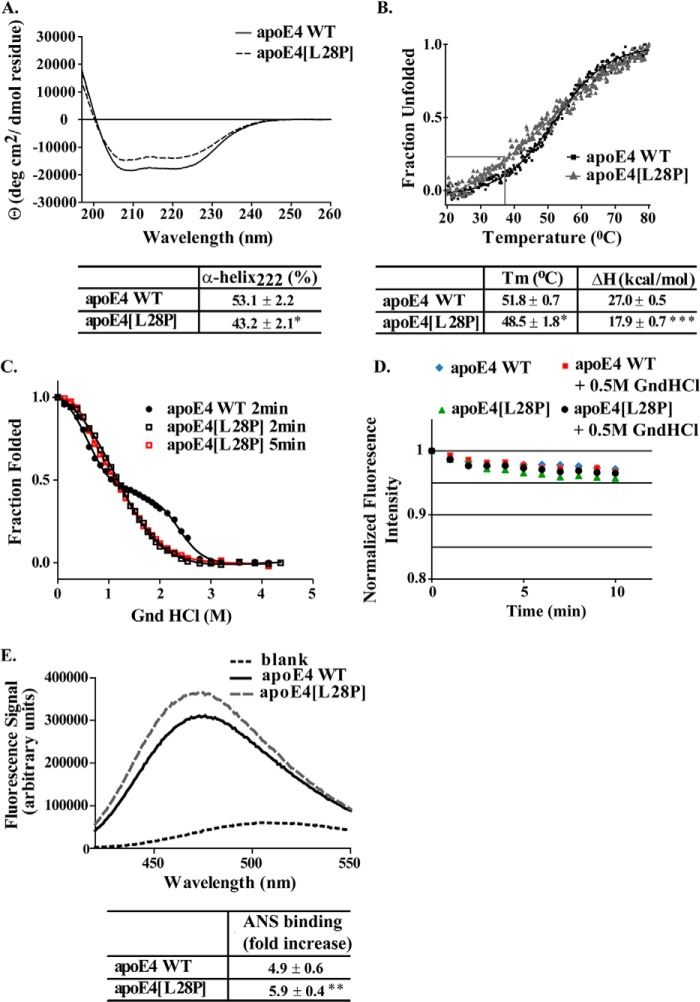
FIGURE 2.
Physicochemical properties of WT apoE4 and apoE4[L28P]. A, far UV CD spectra of WT and mutant apoE4. Spectra are averages of three separate experiments. The percentage of helical content was calculated on the basis of the molar ellipticity at 222 nm, as described under “Experimental Procedure.” B, thermal denaturation profiles of WT and mutant apoE4. The y axis has been normalized to correspond to the fraction of the protein in the unfolded state. Experimental data were fit to a simple two-state Boltzman transition (solid line). Gray lines indicate the portion of the apoE4[L28P] mutant that is in an unfolded state at physiological temperatures (37 °C). Apparent Tm and ΔH values were calculated as described under “Experimental Procedures.” C, chemical denaturation profiles of WT and mutant apoE4. The titration of WT apoE4 with GndHCl was performed using a 2-min incubation between each measurement, whereas that of apoE4[L28P] was performed using a 2- or 5-min incubation. The y axis has been normalized to correspond to the fraction of the protein remaining in the folded state. Experimental data were fitted to a three-state denaturation model as described under “Experimental Procedures” (solid line). D, time course of the fluorescence signal change of the WT or mutant apoE4 in the absence or presence of GndHCl at a final concentration of 0.5 m. E, ANS fluorescence spectra in the presence or absence of WT and mutant apoE4. Spectra are the average of three separate measurements. The fold increase is the increase in ANS fluorescence in the presence of the protein relative to free ANS in the same buffer. *, p < 0.05 versus WT; **, p < 0.01 versus WT; ***, p < 0.005 versus WT.