Abstract
Carnosine (β-alanyl-L-histidine), described as an enigmatic peptide for its antioxidant, anti-aging and especially antiproliferation properties, has been demonstrated to play an anti-tumorigenic role in certain types of cancer. However, its function in human gastric carcinoma remains unclear. In this study, the effect of carnosine on cell proliferation and its underlying mechanisms were investigated in the cultured human gastric carcinoma cells. The mTOR signaling axis molecules were analyzed in carnosine treated cells. The results showed that treatment with carnosine led to proliferation inhibition, cell cycle arrest in the G0/G1 phase, apoptosis increase, and inhibition of mTOR signaling activation by decreasing the phosphorylation of Akt, mTOR and p70S6K, suggesting that proliferation inhibition of carnosine in human gastric carcinoma was through the inhibition of Akt/mTOR/p70S6K pathway, and carnosine would be a mimic of rapamycin.
Keywords: Carnosine, rapamycin, proliferation, gastric carcinoma cell, mTOR signalling pathway
Introduction
Carnosine (β-alanyl-L-histidine), a naturally occurring dipeptide consisting of β-alanine and histidine, is synthesized by carnosine synthetase. As an endogenous substance, it is highly concentrated in muscle and brain tissues, and also presents in other organs such as lungs, kidney and stomach1. Since the discovery of carnosine, repeated clinical efforts have been consistently made to determine its biological functions. And several physiological functions have been pointed out, including antioxidant activity, neurotransmitter function, anti-inflammatory and anti-senescence properties1-5. So, it is not surprising that the enigmatic peptide is increasingly found to be implicated in an increasing number of pathological conditions. For example, it is related to tumors. It has been reported that carnosine regulated some events that contribute to the tumor growth and metabolism, highlighting the critical importance of carnosine's anti-tumorigenic effect6-9. Although it has been suggested that carnosine is involved in cell proliferation, cell cycle arrest, cell apoptosis, and even the glycolytic energy metabolism of certain tumor cells, the molecular mechanisms of the antineoplastic activities of this dipeptide are still not completely understood4,10.
Rapamycin, also known as sirolimus, is a macrolide antibiotic with immunosuppressive and antiproliferative properties, and has been approved as an immunosuppressant drug for organ transplantation, as well as anti-tumor agent for certain cancers11,12. mTOR, the mammalian target of rapamycin, is a serine/threonine downstream mediator in phosphatidylinositol 3-kinase signaling pathway12, and could directly phosphorylate the translational regulator eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 and S6 kinase (p70S6K)13. The mTOR signaling pathway senses and integrates a variety of environmental cues to regulate organismal growth and homeostasis, and plays a critical role in regulating important cellular functions, including proliferation, growth, survival and mobility13-15. Some of the targets of the mTOR kinase, as well as the proteins regulating mTOR, are often been activated or mutated in many types of cancer. In particular, the overexpression of phospho-mTOR has been suggested as an independent prognostic factor of human gastric cancer15,16. Consequently, inhibiting the activation of mTOR axis signaling has become an attractive cancer therapeutic strategy, which merits greater attention.
Interestingly, Hipkiss years ago reviewed that both carnosine and rapamycin could delay ageing, behave as anti-tumor agents, inhibit glycolytic activity, stimulate proteolysis and modulate protein synthesis at the translational level, particularly, both could inhibit translation initiation via effects on eIF4E function17. These phenomena led the author to envision that carnosine might be a rapamycin mimic. Whether similar mechanisms of carnosine and rapamycin on the anti-tumorigenic effect have not yet been addressed. Additionally, the mimic of carnosine to rapamycin is pure speculative and needs further studies. Thus, this study sheds light on the function of carnosine on human gastric carcinoma and its underlying mechanisms. Furthermore, the effects of carnosine on the status of Akt/mTOR/p70S6K signaling were identified.
Materials and methods
Chemicals and reagents
RPMI-1640 medium, fetal bovine serum (FBS), trypsin-EDTA, penicillin and streptomycin were purchased from Biowest (Maine et Loire, France). Carnosine and Rapamycin were obtained from Sigma (St. Louis, MO, USA). The cell counting kit-8 was purchased from Dojindo (Kumamoto, Japan). 2',7'-dichlorofluorescin diacetate (DCFH/DA), the BCA protein assay kit, the Annexin V-FITC apoptosis detection kit and the cell cycle detection kit were purchased from Beyotime (Jiangsu, China). All primary antibodies used in this study were purchased from Cell Signaling Technologies Inc. (Beverly, MA, USA), including p21 (#2947), Cyclin A (#4656), Cyclin E (#4132), Bcl-2 (#2870), Bad (#9239), c-PARP (#9548), Akt (#4685), p-Akt (#4058), mTOR (#2983), p-mTOR (#2971), p70S6K (#2708) and p-p70S6K (#9234). The chemoluminescence reagent was obtained from Millipore (Billerica, MA, USA). All other chemicals were of analytical grade.
Cell line and cell culture
The human gastric carcinoma cell line SGC-7901 was obtained from the Cell Bank of Type Culture Collection Chinese Academy of Sciences (Shanghai), and MKN45 was purchased from Riken Bio Resource Centre. All of the cells were maintained in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin sulphate, and 1 mM sodium pyruvate at 37°C in 5% CO2. The confluent cells were passaged with trypsin-EDTA (0.05% trypsin and 0.53 mM tetrasodium EDTA).
Cell viability assay
The gastric carcinoma cells (SGC-7901 and MKN45) were seeded into 96-well culture plates at a density of 2×103 cells in a final volume of 100 μl/well. After 24 h, the samples of subconfluent cells were treated with various concentrations of carnosine (12.5, 25, 50, 100 and 200 mM) for 24, 48 and 72 h, respectively. Then, the cell growth was determined by cell counting kit-8 assay according to the manufacture's protocol. Experiments were performed in triplicate.
Detection of the cell cycle stage
SGC-7901 cells (3×105) and MKN45 cells (2×105) were plated in 6-well plates and were maintained in fresh culture medium overnight. The following day, the cells were treated with carnosine (50 and 100 mM) and rapamycin (20 nM) for 24 h, respectively. To determine the cell cycle distribution, the gastric carcinoma cells were collected after drug treatment and fixed in 70% ethanol at 4℃ overnight. Then, the cells were labeled with PI (1 mg/ml) in the presence of 1% RNase A for 30 min. The fractions of the cells in G0/G1, S, and G2/M phase were analysed by flow cytometry at an excitation wavelength of 488 nm and an emission wavelength of 630 nm.
Cell apoptosis analysis
The percentage of apoptotic cells was measured using an Annexin V-FITC apoptosis detection kit, according to the manufacturer's protocol. In short, the SGC-7901 and MKN45 cells were treated carnosine (50 and 100 mM) and rapamycin (20 nM) for 24 h, respectively. Subsequently, the cells were harvested and washed twice with PBS, and then resuspended in 200 μl binding buffer containing 2.5 μl Annexin V-FITC. After incubation away from light for 10-15 min at room temperature, the cells were centrifuged and resuspended again in 300 μl binding buffer containing 5 μl of PI. The stained cells were then analysed using a flow cytometer.
Western blot analysis
Western blot analysis was performed as described previously18. Briefly, after the SGC-7901 and MKN45 cells were seeded into 6-well plates and treated with carnosine or rapamycin for 48 h, respectively, the cells were lysed in RIPA lysis buffer containing PMSF for 30 min on ice, followed by centrifugation at 12,000 g for 5 min at 4°C. The supernatant was harvested, and the protein concentration was quantified using a BCA protein assay kit. Equal amounts of protein were separated using SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes followed by incubation with the appropriate primary and secondary antibodies. The immunocomplexes were visualised using a horseradish peroxidase-conjugated antibody followed by a chemoluminescence reagent and detected on photographic film.
Statistical analysis
All data are expressed as the mean ± SD. Significant differences between two groups were determined by Student's t-test, and P < 0.05 was considered to be statistically significant.
Results
Carnosine inhibits the proliferation of human gastric carcinoma cells
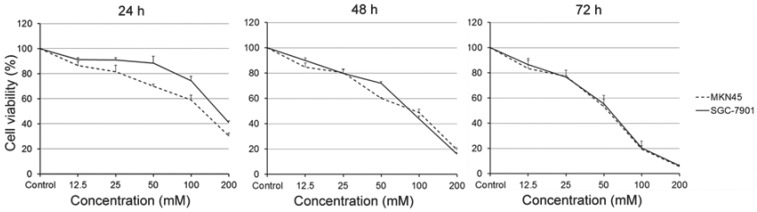
We first examined the viability of gastric carcinoma cells SGC-7901 and MKN45, which treated with carnosine for different concentrations and time points. As expected, the results were similar in two cell lines. Treatment with carnosine at concentrations of 12.5, 25, 50, 100 and 200 mM for the indicated time periods led to a marked concentration- and time-dependent decrease in cell viability. For instance, the cell viability of SGC-7901 cells at the 50 mM carnosine dose was 88% at 24 h, 71% at 48 h and 55% at 72 h, and at the 100 mM dose was 74% at 24 h, 43% at 48 h and 20% at 72 h, respectively (Fig. 1). These data demonstrate that carnosine has a dramatic inhibitory effect on the proliferation of human gastric carcinoma cells.
Fig 1.
Effect of carnosine on the growth of human gastric cancer cell lines. SGC-7901 and MKN45 cells were exposed to different concentrations of carnosine (12.5, 25, 50, 100 and 200 mM) for 24 h, 48 h and 72 h, respectively. The cell growth was determined by cell counting kit-8 assay. The data represent the means ± SD of three independent experiments.
Carnosine triggers cell cycle arrest at G0/G1 phase and regulates the expression of cell cycle-regulating proteins
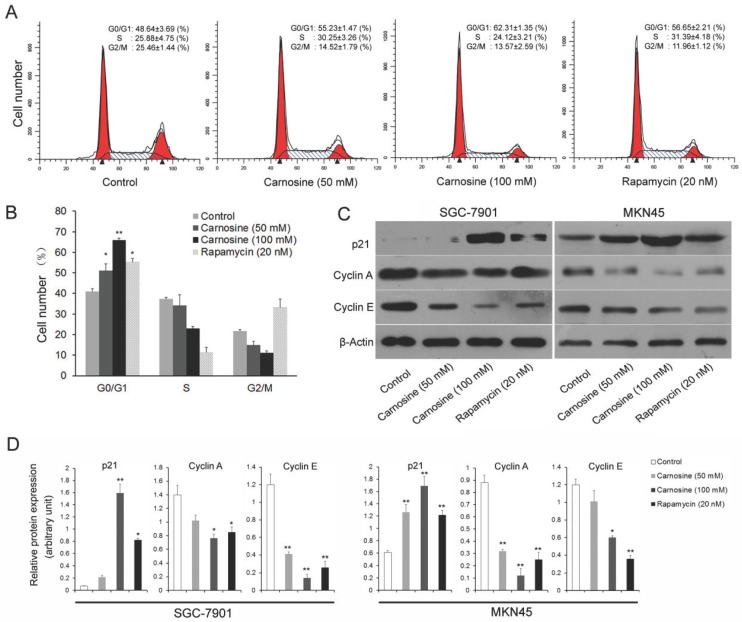
To explore the mechanisms leading to the loss of human gastric carcinoma cells proliferation by carnosine, the effects of carnosine treatment on cell cycle arrest were first examined. Rapamycin, the naturally occurring inhibitor of mTOR, inhibits the growth of cell lines derived from multiple tumor types in vitro, and tumor models in vivo14.Thus, two human gastric carcinoma cell lines were incubated in the presence or absence of carnosine (50 and 100 mM) or rapamycin (20 nM) for 24 h and then analysed by flow cytometry. A dose-dependent G0/G1 arrest in the cell cycle was observed in both cell lines (Fig. 2A, B). The known cytotoxic anti-cancer agent rapamycin resulted in a progressive increase in the population of cells in G0/G1 phase, which was consistent with previous studies19,20. Furthermore, the expression of key molecules that promote the G0/G1 phase transition was assessed. Both cyclin A and cyclin E are critical transition protein which are required for progression from G1 into S phase, and the up-regulation of p21 protein expression is also responsible for the cell cycle arrest21. Western blot analysis showed that carnosine treatment in two cell lines caused a marked dose-dependent increase in the level of p21, whereas the total level of expression of cyclin A and cyclin E were decreased (Fig. 2C, D). These results suggest that inhibition of proliferation of human gastric carcinoma cell lines by carnosine may involve G0/G1 phase arrest of the cell cycle, possibly through alterations of p21 and G0/G1 phase cell cycle-related protein expression.
Fig 2.
Cell cycle analysis of carnosine-treated cell lines. (A) The SGC-7901 cells were treated with carnosine (50 and 100 mM) and rapamycin (20 nM) for 24 h. Then, the cell cycle distribution was measured using flow cytometry. The experiments were performed in triplicate. (B) Cell cycle distribution was analysed by flow cytometry in MKN45 cells 24 h after they were treated with carnosine (50 and 100 mM) and rapamycin (20 nM). The percentage of cells in each population was shown as the mean ± SD from three independent experiments. (C) Western blot analysis of the expression of p21, Cyclin A and Cyclin E in SGC-7901 and MKN45 cells after carnosine and rapamycin treatment for 48 h. (D) The intensity of bands of western blot were quantified by scanning densitometry. Data are means ± SD. *P<0.05, **P<0.01.
Carnosine induces mitochondrial-related apoptosis
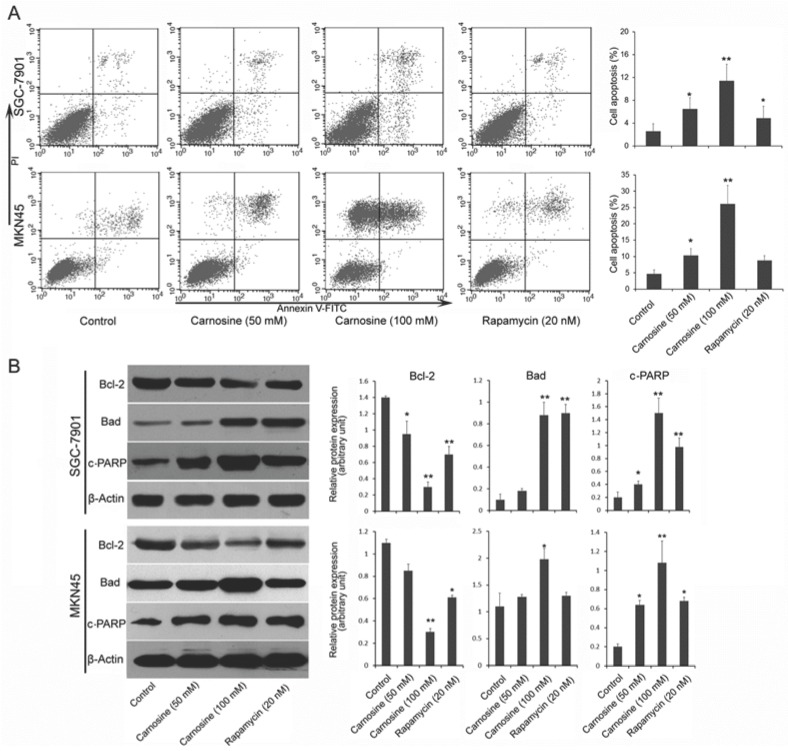
Next, we examined whether and how carnosine induces apoptosis in gastric carcinoma cells. Two cell lines were treated with carnosine (50 and 100 mM) and rapamycin (20 nM) for 24 h, and the apoptotic cells were detected by Annexin V and propidium iodide double staining. As observed in Fig. 3A, treatment with carnosine resulted in a marked dose-dependent increase in apoptosis in both cell lines. Carnosine (at 50 mM concentration) showed a similar effect with rapamycin (20 nM) in SGC-7901 cells, but better than rapamycin in MKN45 cells. To understand the possible mechanism responsible for carnosine-induced apoptotic activity, pivotal proteins associated with apoptosis in the treated cells were examined. The Bcl-2 family, which consists of anti-apoptotic group (Bcl-2, Bcl-xl) and apoptotic group (Bax, Bad), plays a crucial role in regulation of apoptosis through the mitochondrial pathway22. The cleaved PARP (a substrate of caspase-3) is reported to prevent depletion of NAD and ATP that are required for the apoptotic program23. Western blot analysis in two cell lines showed that treatment with carnosine induced a decreased level of anti-apoptotic Bcl-2, but an increased level of apoptotic Bad. Meanwhile, increased cleaved PARP was also observed. Consistently, rapamycin exerted a similar effect on the expression of those proteins (Fig. 3B). These results reveal that carnosine-induced cell apoptosis is partially mediated by the mitochondria pathway and caspase activation.
Fig 3.
Carnosine induces apoptosis in human gastric carcinoma cells. (A) Following treatment of SGC-7901 and MKN45 cells with carnosine (50 and 100 mM) and rapamycin (20 nM) for 24 h, apoptotic cells of the two cell lines were detected by Annexin V and propidium iodide double staining. Statistical analysis of the percentages of the apoptotic cells. (B) Western blots of whole-cell extracts of treated cells were analyzed for Bcl-2, Bad and cleaved-PARP after treatment with carnosine and rapamycin for 48 h. The data shown are representatives of three experiments. *P<0.05, **P<0.01.
Carnosine inhibits the activation of AKT/mTOR signaling pathway
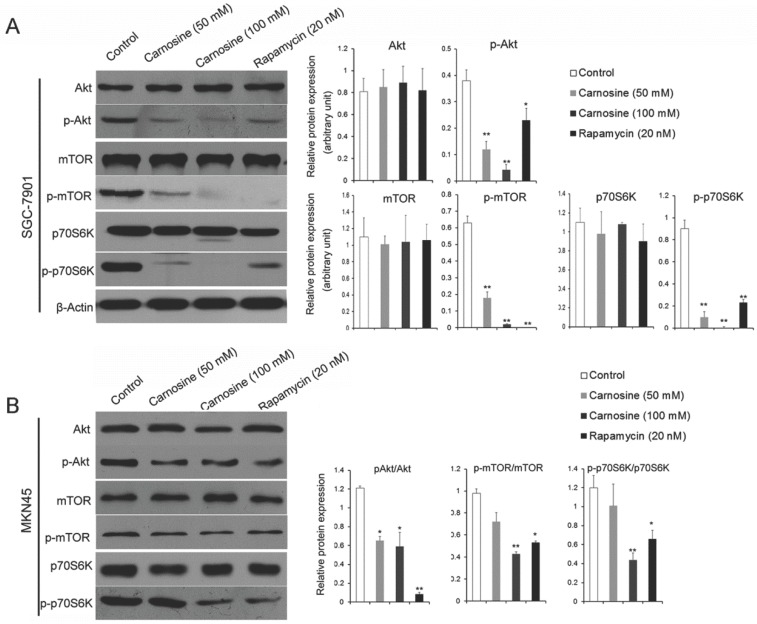
The results described above hint that carnosine mimics some effects of rapamycin. It is suggested that the mTOR-containing complexes have different sensitivities to rapamycin as well as upstream inputs and downstream outputs13. Therefore, the upstream activator Akt and the downstream effector p70S6K were analyzed by western blot in the carnosine or rapamycin treated cells. As shown in Fig. 4A, B, carnosine treatment induced a significantly dose-dependent decrease in the phosphorylation status of Akt, mTOR and p70S6K in both SGC-7901 and MKN45 cells. Similarly, rapamycin influenced the activation of the three biological markers. However, both carnosine and rapamycin failed to alter the total protein levels of Akt, mTOR and p70S6K. Taken together, these findings open the possibility that carnosine, like rapamycin, could inhibit the activation of Akt/mTOR/p70S6K pathway to reduce the proliferation of human gastric cancer in vitro.
Fig 4.
Carnosine reduces the phosphorylation status of in AKT/mTOR pathway. The effect of carnosine on protein markers of AKT/mTOR signaling pathway was assessed by western blot after SGC-7901 (A) and MKN45 (B) cells were treatment with carnosine and rapamycin for 48 h. β-Actin was used as the loading control. The data are means ± SD of three independent experiments. *P<0.05, **P<0.01.
Discussion
In the present study, we demonstrated that carnosine significantly suppressed the growth of human gastric carcinoma cells. And, the dipeptide, similar with rapamycin, triggered cell cycle arrest at G0/G1 phase and induced cell apoptosis through inhibiting the mTOR signaling pathway.
Several observations support the importance of mTOR pathway in cancer pathogenesis13. Preclinical data suggested that suppression of mTOR pathway inhibited the proliferation of gastric cancer cells and delayed tumor progression in in vitro and animal models16. For its antiproliferative effects, rapamycin has the role in treating cancer. Furthermore, as a naturally occurring inhibitor of mTOR, rapamycin has a number of analogs in clinical trials, such as CCI-779 (temsirolimus), RAD-001 (everolimus) and AP23573 (ariad)24,25. These analogs, containing minor chemical modifications to the parent structure, could inhibit the growth of cell lines derived from multiple tumor types and are being tested for use in cancer14. However, diverse lines of evidence reveal that these analogs have a higher rate of fatal side effects in cancer patients and should be combined with other agents to achieve a better treatment for certain types of cancer26-28. Therefore, it is necessary to search for the new rapamycin analog or mimic.
In the current study, treatment with carnosine in human gastric carcinoma cells resulted in G0/G1 phase arrest in a dose-dependent manner, increased the level of p21 and reduced the G1/S transition related proteins: cyclin A and cyclin E. This is in agreement with the evidence that carnosine induces G1 arrest in HCT116 cells by inhibiting the G1/S phase transition6. Additionally, Jia et al also proved that carnosine could inhibit mesangial cell proliferation by modulating cell cycle progress7. So, it is plausible that alterations in cell cycle associated proteins may lead to the arrest of G0/G1 phase in carnosine treated cells.
Emerging evidence has discussed the protective effect of carnosine extensively. In vivo, carnosine was shown to prevent testicular dysfunction induced by gamma-irradiation via an anti-apoptotic effect and decrease reactive oxygen species levels and preserve normal glutathione levels in the ischemic brain29,30. Moreover, in human neuroblastoma cell line SH-SY5Y, carnosine was proved to almost completely inhibit 6-OHDA-induced ER stress responses and cytotoxicity31. Nevertheless, carnosine has been suggested having the benefit of supressing the growth of tumor. For instance, some reports indicated that carnosine retarded tumor growth in the NIH3T3-HER2/neu and HCT116 cells implanted BALB/c nude mice model8,9. Here, we also showed that following carnosine treatment, a dose dependent apoptosis was observed, and the expression of cleaved-PARP and ratio of Bcl-2/Bad were both increased. Concerning the effect of rapamycin on the expression of Bad, Liu et al. demonstrated that the phosphorylation of Bad was enhanced under the treatment of rapamycin, but not the total amount of the protein32. However, our results and some other more reports suggested that rapamycin could influence the total level of Bad33,34. Notwithstanding, our findings suggest that carnosine could influence the growth of human tumor, consistent with the previous data which showing carnosine inhibits the growth of human glioma cell by inhibiting ATP production35.
The discrepant properties of carnosine towards normal tissues and tumor may be involved in the different mechanisms, and future studies are needed to explore the discrepancy. Regardless, under the achieved background of our results and others, one could conclude that carnosine would render an effective pharmacological treatment of human neoplasia, at least to some extent. Up to now, the physiological and biochemical mechanisms responsible for the anti-neoplastic activity of carnosine are not clear1. Although possible mechanisms of carnosine in tumor have been extensively studied, such as influencing the chaperone activity and hypoxia inducible factor alpha signalling36, reacting with AGEs37, scavenging reactive oxygen species38, and inhibiting phosphorylation of ERK 1/2 and p38 MAP kinase1, few investigations have focused on the effects of carnosine on mTOR signaling pathway. Thus, further experiments in this study were performed in order to understand how carnosine affects the mTOR axis proteins at the molecular level. The data showed that carnosine, compared with rapamycin, strongly inhibited p70S6K phosphorylation, and simultaneously inhibited the phosphorylation of Akt, which is the key event regulating the ribosomal S6 kinase (S6K) phosphorylation in the mTOR pathway39. And it is thought that p70S6K promoted cell cycle arrest and regulated cell survival by affecting the expression of related molecules. Additionally, in parallel with our current results, Son et al demonstrated that the suppression of Akt phosphorylation by carnosine could inhibit 4E-BP phosphorylation and lead to the suppression of eIF4F complex formation, resulting in the inhibition of mTOR signaling pathway1. Moreover, it was suggested that branched-chain amino acids enhance premature senescence through mTOR signalling pathway mediated upregulation of p21 protein40. And the increased expression of p21 is frequently accompanied with the mTOR inhibition41. These studies, along with our results showing reduced levels of p-Akt, -TOR, -p70S6K in gastric carcinoma cells treated with carnosine, strongly indicate that Akt/mTOR/p70S6K signaling cascade is one of the major targets of carnosine.
In conclusion, the present study describes a new possible molecular mechanism of the antitumor effects of carnosine in human gastric carcinoma cells. Carnosine strongly inhibits the growth of cultured tumor cells, arrests the cell cycle at G0/G1 phase through alerting p21 and cell cycle-related protein expression, and induces the mitochondrial-related apoptosis partially mediated by the mitochondria pathway and caspase activation. Moreover, the dipeptide, similar with rapamycin, inhibits the activation of Akt, mTOR and p70S6K protein. Thus, it seems reasonable to conclude that proliferation inhibition of carnosine in human gastric carcinoma is performed through the inhibition of Akt/mTOR/p70S6K pathway and the peptide would be a mimic of rapamycin. Our research may add a new dimension to our understanding of the physiological and biochemical mechanisms responsible for the anti-neoplastic activity of carnosine. In addition, manipulation of the activation of mTOR signaling pathway with carnosine may provide novel therapeutic targets for human gastric carcinoma and even other human diseases.
References
- 1.Son DO, Satsu H, Kiso Y. et al. Inhibitory effect of carnosine on interleukin-8 production in intestinal epithelial cells through translational regulation. Cytokine. 2008;42:265–76. doi: 10.1016/j.cyto.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Fontana M, Pinnen F, Lucente G. et al. Prevention of peroxynitrite-dependent damage by carnosine and related sulphonamido pseudodipeptides. Cell Mol Life Sci. 2002;59:546–51. doi: 10.1007/s00018-002-8446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadi NS, Hirsch JD, Margolis FL. Laminar distribution of putative neurotransmitter amino acids and ligand binding sites in the dog olfactory bulb. J Neurochem. 1980;34:138–146. doi: 10.1111/j.1471-4159.1980.tb04632.x. [DOI] [PubMed] [Google Scholar]
- 4.Gaunitz F, Hipkiss AR. Carnosine and cancer: a perspective. Amino Acids. 2012;43:135–42. doi: 10.1007/s00726-012-1271-5. [DOI] [PubMed] [Google Scholar]
- 5.Gallant S, Semyonova M, Yuneva M. Carnosine as a potential anti-senescence drug. Biochemistry (Mosc) 2000;65:866–8. [PubMed] [Google Scholar]
- 6.Iovine B, Iannella ML, Nocella F. et al. Carnosine inhibits KRAS-mediated HCT116 proliferation by affecting ATP and ROS production. Cancer Lett. 2012;315:122–8. doi: 10.1016/j.canlet.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Jia H, Qi X, Fang S. et al. Carnosine inhibits high glucose-induced mesangial cell proliferation through mediating cell cycle progression. Regul Pept. 2009;154:69–76. doi: 10.1016/j.regpep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Renner C, Zemitzsch N, Fuchs B. et al. Carnosine retards tumor growth in vivo in an NIH3T3-HER2/neu mouse model. Mol Cancer. 2010;9:2. doi: 10.1186/1476-4598-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horii Y, Shen J, Fujisaki Y. et al. Effects of l-carnosine on splenic sympathetic nerve activity and tumor proliferation. Neurosci Lett. 2012;510:1–5. doi: 10.1016/j.neulet.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 10.Sale C, Artioli GG, Gualano B. et al. Carnosine: from exercise performance to health. Amino Acids. 2013;44:1477–91. doi: 10.1007/s00726-013-1476-2. [DOI] [PubMed] [Google Scholar]
- 11.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 12.Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer. 2004;91:1420–4. doi: 10.1038/sj.bjc.6602162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–46. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 15.Yu G, Wang J, Chen Y. et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer. Clin Cancer Res. 2009;15:1821–9. doi: 10.1158/1078-0432.CCR-08-2138. [DOI] [PubMed] [Google Scholar]
- 16.Al-Batran SE, Ducreux M, Ohtsu A. mTOR as a therapeutic target in patients with gastric cancer. Int J Cancer. 2012;130:491–6. doi: 10.1002/ijc.26396. [DOI] [PubMed] [Google Scholar]
- 17.Hipkiss AR. Energy metabolism, proteotoxic stress and age-related dysfunction - protection by carnosine. Mol Aspects Med. 2011;32:267–78. doi: 10.1016/j.mam.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Miao L, Lv C. et al. Wentilactone B induces G2/M phase arrest and apoptosis via the Ras/Raf/MAPK signaling pathway in human hepatoma SMMC-7721 cells. Cell Death Dis. 2013;4:e657. doi: 10.1038/cddis.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cejka D, Preusser M, Woehrer A. et al. Everolimus (RAD001) and anti-angiogenic cyclophosphamide show long-term control of gastric cancer growth in vivo. Cancer Biol Ther. 2008;7:1377–85. doi: 10.4161/cbt.7.9.6416. [DOI] [PubMed] [Google Scholar]
- 20.Xu DZ, Geng QR, Tian Y. et al. Activated mammalian target of rapamycin is a potential therapeutic target in gastric cancer. BMC Cancer. 2010;10:536. doi: 10.1186/1471-2407-10-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huet X, Rech J, Plet A. et al. Cyclin A expression is under negative transcriptional control during the cell cycle. Mol Cell Biol. 1996;16:3789–98. doi: 10.1128/mcb.16.7.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliver FJ, Menissier-de Murcia J, de Murcia G. Poly (ADP-ribose) polymerase in the cellular response to DNA damage, apoptosis, and disease. Am J Hum Genet. 1999;64:1282–8. doi: 10.1086/302389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan X, Shen N, Mendoza A. et al. CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling. Neoplasia. 2006;8:394–401. doi: 10.1593/neo.05820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemoine M, Derenzini E, Buglio D. et al. The pan-deacetylase inhibitor panobinostat induces cell death and synergizes with everolimus in Hodgkin lymphoma cell lines. Blood. 2012;119:4017–25. doi: 10.1182/blood-2011-01-331421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiarini F, Lonetti A, Teti G. et al. A combination of temsirolimus, an allosteric mTOR inhibitor, with clofarabine as a new therapeutic option for patients with acute myeloid leukemia. Oncotarget. 2012;3:1615–28. doi: 10.18632/oncotarget.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabinski N, Ewald F, Hofmann BT. et al. Combined targeting of AKT and mTOR synergistically inhibits proliferation of hepatocellular carcinoma cells. Mol Cancer. 2012;11:85. doi: 10.1186/1476-4598-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai S, Sun Y, Liu N. et al. Combination of Rad001 (everolimus) and propachlor synergistically induces apoptosis through enhanced autophagy in prostate cancer cells. Mol Cancer Ther. 2012;11:1320–31. doi: 10.1158/1535-7163.MCT-11-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haeri SA, Rajabi H, Fazelipour S. et al. Carnosine mitigates apoptosis and protects testicular seminiferous tubules from gamma-radiation-induced injury in mice. Andrologia. 2013 doi: 10.1111/and.12193. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Rajanikant GK, Zemke D, Senut MC. et al. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke. 2007;38:3023–31. doi: 10.1161/STROKEAHA.107.488502. [DOI] [PubMed] [Google Scholar]
- 31.Oh YM, Jang EH, Ko JH. et al. Inhibition of 6-hydroxydopamine-induced endoplasmic reticulum stress by l-carnosine in SH-SY5Y cells. Neurosci Lett. 2009;459:7–10. doi: 10.1016/j.neulet.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Sun SY, Owonikoko TK. et al. Rapamycin induces Bad phosphorylation in association with its resistance to human lung cancer cells. Mol Cancer Ther. 2012;11:45–56. doi: 10.1158/1535-7163.MCT-11-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng J, Hudder A, Zukowski K. et al. Rapamycin sensitizes Akt inhibition in malignant human breast epithelial cells. Cancer Lett. 2010;296:74–87. doi: 10.1016/j.canlet.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhuri J, Chowdhury AA, Biswas N. et al. Superoxide activates mTOR-eIF4E-Bax route to induce enhanced apoptosis in leukemic cells. Apoptosis. 2014;19:135–48. doi: 10.1007/s10495-013-0904-9. [DOI] [PubMed] [Google Scholar]
- 35.Renner C, Asperger A, Seyffarth A. et al. Carnosine inhibits ATP production in cells from malignant glioma. Neurol Res. 2010;32:101–5. doi: 10.1179/016164109X12518779082237. [DOI] [PubMed] [Google Scholar]
- 36.Asperger A, Renner C, Menzel M. et al. Identification of factors involved in the anti-tumor activity of carnosine on glioblastomas using a proteomics approach. Cancer Invest. 2011;29:272–81. doi: 10.3109/07357907.2010.550666. [DOI] [PubMed] [Google Scholar]
- 37.Hipkiss AR. On the enigma of carnosine's anti-ageing actions. Exp Gerontol. 2009;44:237–42. doi: 10.1016/j.exger.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Guiotto A, Calderan A, Ruzza P. et al. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12:2293–315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- 39.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Nakano M, Nakashima A, Nagano T. et al. Branched-chain amino acids enhance premature senescence through mammalian target of rapamycin complex I-mediated upregulation of p21 protein. PLoS One. 2013;8:e80411. doi: 10.1371/journal.pone.0080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin HO, Hong SE, Woo SH. et al. Silencing of Twist1 sensitizes NSCLC cells to cisplatin via AMPK-activated mTOR inhibition. Cell Death Dis. 2012;3:e319. doi: 10.1038/cddis.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]