Key Points
Image-guided intrathymic injection of cells or drugs permits implementation of clinically relevant strategies to improve thymic function.
Intrathymic injection of hematopoietic stem cells generates long-lasting antigen-specific T-cell immunity.
Abstract
T-cell deficiency related to disease, medical treatment, or aging represents a major clinical challenge and is associated with significant morbidity and mortality in cancer and bone marrow transplantation recipients. This study describes several innovative and clinically relevant strategies to manipulate thymic function based on an interventional radiology technique for intrathymic injection of cells or drugs. We show that intrathymic injection of multipotent hematopoietic stem/progenitor cells into irradiated syngeneic or allogeneic young or aged recipients resulted in efficient and long-lasting generation of functional donor T cells. Persistence of intrathymic donor cells was associated with intrathymic presence of cells resembling long-term hematopoietic stem cells, suggesting a self-renewal capacity of the intrathymically injected cells. Furthermore, our approach enabled the induction of long-term antigen-specific T-cell–mediated antitumor immunity following intrathymic injection of progenitor cells harboring a transgenic T-cell receptor gene. The intrathymic injection of interleukin-7 prior to irradiation conferred radioprotection. In addition, thymopoiesis of aged mice improved with a single intrathymic administration of low-dose keratinocyte growth factor, an effect that was sustained even in the setting of radiation-induced injury. Taken together, we established a preclinical framework for the development of novel clinical protocols to establish lifelong antigen-specific T-cell immunity.
Introduction
Cancer patients and in particular bone marrow transplantation (BMT) recipients are at risk for the development of prolonged T-cell deficiency associated with cytotoxic therapies or immunosuppressive treatments. These patient populations would greatly benefit from protocols boosting antigen-specific T-cell immunity.1 The organ of T-cell development, the thymus, is therefore an obvious target organ for immunotherapeutic strategies, yet thymus-based approaches have traditionally been underutilized in clinical protocols to improve immunity. A main reason for this lack of thymus-centered trials has to do with the fact that thymic cell populations are highly sensitive and prone to injury. In addition, bone marrow (BM)-derived circulating thymus-seeding T-cell progenitors are of critical importance for proper thymic function, and the lack thereof, such as during periods of lymphopenia, compromises thymopoiesis.2 As such, overcoming reduced thymic function, be it due to medical conditions or treatments, stress, or age-related thymic involution,3,4 is always challenging, and clinical protocols designed to boost endogenous thymic function by administration of thymopoietic growth factors5 have been only mildly successful to date. Protocols for adoptive transfer of naïve or antigen-specific T cells have been developed in an attempt to boost T-cell immunity, but persistence of transferred T cells can be an issue,1 and availability of suitable donor cells can be a major limiting factor. Therefore, there is clearly a need for alternate clinically feasible strategies to improve thymopoiesis and T-cell immunity. Our study demonstrates that image-guided injection of the thymus with cells or drugs presents promising opportunities for clinical immunotherapy.
Methods
Mice, BMT, irradiation, and tumor challenge
We obtained female C57BL/6 (B6, H-2b), C57BL/6 (CD45.1+) (H-2b), BALB/c (H-2d) mice from The Jackson Laboratory. C57BL/6.Luc+.Thy1.1+ transgenic mice, which express firefly luciferase under the control of the widely expressed β-actin promoter, were obtained from Robert Negrin (Stanford University).6 Pmel-1 T-cell receptor (TCR) transgenic mice7 (a gift from N. Restifo, National Cancer Institute) were bred with Thy1.1+ C57BL/6 mice (The Jackson Laboratory) as a source of tumor-specific lineage marker−Sca-1+c-kit+ (LSK) cells. All mice were maintained at Memorial Sloan-Kettering Cancer Center in accordance with Institutional Animal Care and Use Committee standards.
Sublethal irradiation or BMT experiments were performed with single-dose TBI of BALB/c recipients (550 cGy or 700 cGy) or C57BL/6 recipients (700 cGy or 850 cGy) from a 137Cs source. BMT recipients were transplanted with intravenously injected BM cells (5-10 × 106) that were removed aseptically from femurs and tibias followed by T-cell depletion with anti-Thy-1.2 and low-TOX-M rabbit complement (Cedarlane Laboratories, Burlington, NC).
Thymic irradiation (3300 cGy) was performed with a X-RAD 320 orthovoltage energy X-ray unit (Precision X-ray, North Branford, CT). Animals were shielded with a lead shield allowing radiation exposure of the thymic area only.
In graft-versus-tumor experiments, animals received 50 000 B16 F10 melanoma cells (H-2b) (a gift from I. Fidler, M.D. Anderson Cancer Center, Houston, TX) on day 28 after BMT via intradermal injection into the shaved right flank. Tumor diameters were measured with a caliper, and mice were sacrificed when the diameter exceeded 1 cm, tumors became ulcerated, or mice showed discomfort.
Ultrasound-guided intrathymic injections
Mice were anesthetized using continuous administration of 1% to 4% isoflurane anesthesia via a nose cone and precision calibrated vaporizer. After induction of anesthesia, mice were positioned supine on a heated 37°C animal platform of the Vevo Imaging Station (VisualSonics, Toronto, Ontario, Canada). Mice were secured to the stage by applying transparent medical adhesive tape (Covidien, Mansfield, MA) to their hindlimbs and forelimbs. The previously epilated skin of the upper thorax was sterilely prepared using Chloraprep One-Step (CareFusion, Leawood, KS), a chlorhexidine gluconate wash. Ultrasound imaging was performed with the Vevo2100 (VisualSonics) using a MS-550S 40 mHz linear transducer using an imaging depth of 6 to 7 mm. After the probe was sheathed with a sterile latex cover (Sheathing Technologies, Morgan Hill, CA), sterile Aquasonic 100 gel (Parker Laboratories, Fairfield, NJ) was then applied to the transducer to produce several millimeters of stand-off during imaging. While sterilely gloved, the radiologist moved the needle of a 30G insulin syringe (BD, Franklin Lakes, NJ) containing 10 μL of injectate in the stand-off gel under the transducer until it was adequately visualized at the skin surface, and then it was inserted into the thymus gland via a percutaneous trajectory away from blood vessels. Once the needle tip was within the thymus, syringe contents were injected during sonographic visualization.
In vivo BLI
Intravital monitoring of intrathymically injected LSK cells was performed by in vivo bioluminescence imaging (BLI). Mice received d-luciferin (3 mg/mouse; Goldbio, St. Louis, MO) intraperitoneally and were anesthetized by isoflurane and imaged 15 minutes after injection using an IVIS-200 imaging system (PerkinElmer, Waltham, MA). Pseudocolor images showing whole-body distribution of the bioluminescent signal were superimposed on conventional grayscale photographs.
Flow cytometry and cell sorting
Cells were incubated with antibodies at 4°C for 20 minutes and washed with fluorescence-activated cell sorter (FACS) buffer. The stained cells were resuspended in FACS buffer and analyzed on an LSR-II flow cytometer (Becton Dickinson, San Jose, CA) with FACSDiva software (Becton Dickinson). Data were analyzed with FlowJo (Tree Star, Ashland, OR). Lineage-marker–negative BM was obtained using a lineage depletion kit (Miltenyi Biotec, San Diego, CA), followed by isolation of LSK cells. For separation of LSK cells, mouse lineage-depleted BM cells were incubated with a mix of fluorescein isothiocyanate–conjugated lineage antibodies (anti-CD3, NK1.1, Gr-1, CD11b, CD11c, CD19, CD4, and CD8) and with anti-Sca-1 and anti-c-kit antibodies. LSK cells were isolated using a FACSAriaII cell sorter (Becton Dickinson).
Reagents and antibodies
All of the following monoclonal antibodies against murine antigens were obtained from BD Biosciences (San Jose, CA): CD45.1 (clone A20), Thy1.1 (clone OX-7), H-2Dd (clone 34-2-12), CD3 (clone 145-2C11), CD4 (clone RM4-5), CD8a (clone 53-6.7), CD19 (clone 1D3), CD62L (clone MEL-14), CD44 (clone IM7), NK1.1 (clone PK136), CD11c (clone HL3), CD11b (clone M1/70), Gr-1 (clone RB6-8C5), CD25 (clone PC61), interleukin (IL)-2 (clone JES6-5H4), interferon-γ (IFN-γ) (clone XMG1.1), mouse Vβ TCR screening panel, c-kit (clone 2B8), Sca-1 (clone D7), rat immunoglobulin G2b-κ (clone A95-1), and rat immunoglobulin G1-κ (clone R3-34). Antibodies targeting mouse B220 (clone RA3-6B2), CD150 (clone TC15), and CD48 (clone HM48-1) were purchased from BioLegend (San Diego, CA). A mouse IL-7-Rα–specific antibody (clone A7R43) was obtained from eBioscience (San Diego, CA). Gp100 iTAg major histocompatibility complex tetramer was purchased from Medical and Biological Laboratories (Nagoya, Japan). A fixation/permeabilization solution kit was also obtained from BD Biosciences. 4,6 Diamidino-2-phenylindole (Molecular Probes, Eugene, OR) was used for dead cell discrimination. Ionomycin and 4-phorbol 12-myristate 13-acetate were obtained from Calbiochem (La Jolla, CA). GolgiPlug was obtained from BD Biosciences. Recombinant mouse IL-7 and recombinant mouse IL-22 were purchased from Miltenyi Biotec, and mouse recombinant keratinocyte growth factor (KGF) was purchased from R&D Systems (Minneapolis, MN).
Statistics
All results are based on 2-sided test statistics. A P value < .05 was considered statistically significant. The Mann-Whitney U statistic was used to compare data between 2 groups.
Results
Intrathymic injection of multipotent stem cells produces functional donor T cells
In targeting the need for additional clinically feasible strategies improving thymopoiesis and T-cell immunity, we developed a minimally invasive interventional radiology method for aseptic intrathymic injection (ITI) based on ultrasound-guided free-hand injection (see supplemental Figure 1 available at the Blood Web site). Due to the unique capacity of the thymic microenvironment to impose a T-cell lineage fate on almost all hematopoietic progenitor cells with multilineage potential,8 ITI of BM-derived hematopoietic stem/progenitor cells (HSPCs) holds significant promise for immunotherapy and clinical translation. We first determined if multipotent HSPCs, defined as LSK cells, or more committed lymphoid-primed multipotent progenitor cells, an LSK subset representing the presumed BM equivalent of circulating T-cell progenitors,9 are more effective in repopulating the thymus upon injection into an irradiated recipient. We found that unfractionated LSK cells were superior to purified lymphoid-primed multipotent progenitor cells (data not shown) and therefore used LSK cells as the donor cell source for our subsequent experiments. Next, we established the minimum cell dose needed for sufficient T-cell reconstitution from LSK cells that were intrathymically injected into sublethally irradiated recipients. We found that as little as 1000 LSK cells were sufficient to generate donor T cells in this setting but injection of 2000 cells had a superior overall effect (supplemental Figure 2). We therefore used cell doses of at least 2000 LSK cells (ranging from 3000 to 10 000 cells per injection) for all subsequent experiments. In order to establish a clinically applicable cell therapy strategy, we decided to first focus on ITI of syngeneic HSPCs (equivalent to autologous CD34+ human HSPCs). To facilitate engraftment, recipients were exposed to sublethal irradiation (usually in the form of total body irradiation [TBI]). We found that when intrathymically injected 2 to 3 hours after sublethal TBI, syngeneic HSPCs had excellent thymus-repopulating capacity (Figure 1A-C) and resulted in robust peripheral T-cell reconstitution (Figure 1D). Overall thymic cellularity was not increased compared with irradiated controls (data not shown). More than 90% progeny of intrathymically injected HSPCs differentiated into T cells (Figure 1E) that were characterized by a normal CD4 to CD8 ratio (Figure 1E), predominance of naive and central memory phenotypes (Figure 2A), appropriate cytokine production in response to nonspecific stimulation with a strong mitogen (Figure 2B), and a diverse TCR repertoire (Figure 2C). Of note, thymic conditioning with focal thymic irradiation was as effective as sublethal TBI, whereas injection of syngeneic HSPCs into the thymus of nonconditioned mice resulted in only minimal donor T-cell reconstitution, indicating that even in the absence of rejection the number of available precursor cell niches is still a limiting factor (Figure 2D).
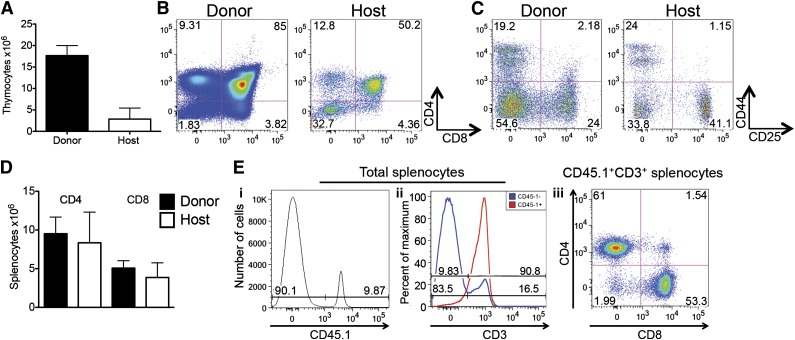
Figure 1.
ITI of multipotent stem cells results in efficient T-cell generation in sublethally irradiated syngeneic recipients. (A) Sublethally irradiated (700 cGy) C57BL/6 recipients received 6000 C57BL/6.CD45.1+ LSK cells via ITI 2 to 3 hours after irradiation. Thymuses were harvested on day 60 after radiation and analyzed for host and LSK donor origin. Mean and standard error of the mean (SEM) are presented (n = 4). (B) Animals were treated as described in panel A. Thymuses were harvested on day 60 after radiation and analyzed for CD4 and CD8 DP and SP populations of host and LSK donor origin. Representative plots of 1 of 4 samples are presented. (C) Animals were treated as described in panel A. Thymuses were harvested on day 60 after radiation and analyzed for CD4 and CD8 DN subsets of host and LSK donor origin. Representative plots of 1 of 4 samples are presented. (D) Animals were treated as described in panel A. Spleens were harvested on day 60 after radiation and analyzed for host and donor origin of CD4+ and CD8+ T cells. Mean and SEM are presented (n = 4). (E) Animals were treated as described in panel A. Spleens were harvested on day 60 after radiation. (i) Splenocytes were analyzed for donor and host origin. (ii) Percentage of T cells in host and donor splenocytes. (iii) Percentage of CD4+ and CD8+ cells in donor T cells. Representative histograms/plots of 1 of 4 samples are presented.
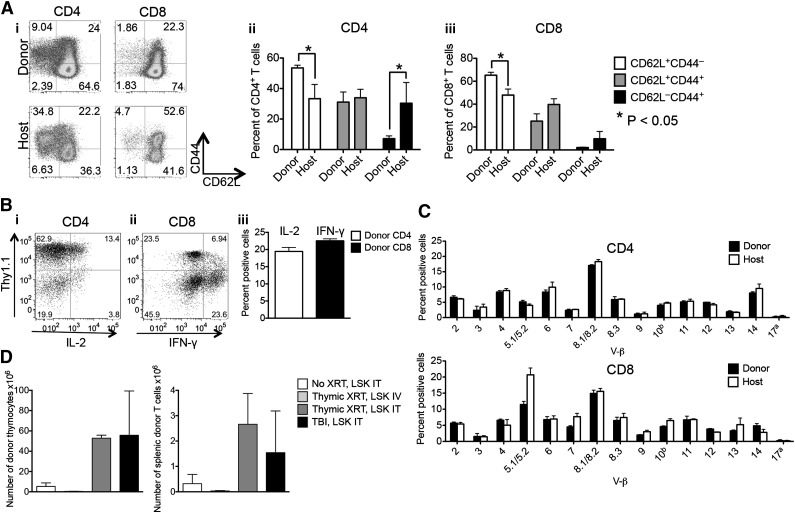
Figure 2.
Intrathymically injected HSPCs give rise to functional T cells. (A) Sublethally irradiated (700 cGy) C57BL/6 recipients received 6000 C57BL/6.CD45.1+ LSK cells via ITI 2 to 3 hours after irradiation. (i-iii) Percentage of naïve, central, and effector memory CD4+ and CD8+ T cells of host and donor origin. (i) A representative plot of 1 of 4 samples is presented. (ii-iii) Mean and SEM are presented (n = 4). (B) Sublethally irradiated C57BL/6 recipients received 5000 C57BL/6.Thy1.1+ LSK cells via ITI 2 hours after irradiation. Splenocytes were harvested on day 60 after radiation and were stimulated with 4-phorbol 12-myristate 13-acetate/ionomycin followed by intracellular staining for IL-2 and IFN-γ. CD4+ and CD8+ T cells of donor origin were gated on IL-2+ and IFN-γ+ cells. (i-ii) Representative plots of one of 4 samples are presented. (iii) Mean and SEM are presented (n = 4). (C) Animals were treated as described in panel B. Splenocytes were harvested on day 60 after radiation and were stained with V-β screening panel, and the TCR repertoire of CD4+ and CD8+ donor and host T cells was analyzed. Mean and SEM are presented (n = 4). (D) C57BL/6 recipients received 3000 C57BL/6.CD451.1+ LSK cells via ITI 2 hours after sublethal TBI or thymic irradiation or in the absence of radiation. In an additional group, C57BL/6 recipients received C57BL/6.CD45.1+ LSK cells via intravenous injection 2 hours after thymic irradiation. Thymuses and spleens were harvested on day 30 after LSK injection and analyzed for thymocytes and splenic T cells of donor origin. Mean and SEM are presented (n = 4). IT, intrathymic; IV, intravenous; XRT, radiation therapy.
ITI of HSPCs generates long-lasting donor T cells after BMT
We next evaluated the fate of intrathymically injected HSPCs in the setting of BMT. Syngeneic or allogeneic recipients were exposed to lethal TBI, followed by intravenous administration of T-cell–depleted BM as well as ITI of HSPCs that were congenic with the BM donor. Consistent with previous studies, we found efficient and long-lasting T-cell reconstitution in the settings of both syngeneic and allogeneic BMT (Figure 3A-F) from intrathymically injected HSPCs.10-12 Total thymic cellularity and splenic donor T-cell numbers were not significantly altered as a result of ITI of HSPCs. In some cases, it appeared that the generation of ITI-derived T cells of LSK donor origin occurred at the expense of BM donor-derived T cells (2 months after syngeneic BMT, Figure 3B; 6 months after allogeneic BMT, Figure 3H). Notably, the phenotype of ITI-derived thymocytes following BMT resembled the phenotype associated with physiologic thymopoiesis (predominance of double-positive [DP] thymocytes), in contrast to BM donor-derived thymocytes, which were characterized by a decrease in DP thymocytes following allogeneic BMT (Figure 3E,G). Importantly, in vivo BLI demonstrated persistence of the progeny of intrathymically injected syngeneic HSPCs for up to 15 months (Figure 4A and supplemental Figure 3). Analysis of the earliest stages of T-cell development revealed that intrathymically injected HSPCs had an extraordinary capacity to give rise to early T-cell progenitors (Figure 4B); after syngeneic BMT, we were even able to identify persistence of CD150+CD48− thymocytes resembling long-term hematopoietic stem cells (HSCs) in the BM (Figure 4C), whereas allogeneic BMT was associated with persistence of committed hematopoietic progenitor cells13 (supplemental Figure 4) and lack of persistence of HSCs. The latter phenotypes are not known to exist in the thymus under physiological conditions. We were also able to detect intrathymic myeloid cells (Figure 4D) and dendritic cells (data not shown) derived from intrathymically injected HSPCs, indicating that despite the highly specialized thymic microenvironment, it is possible for injected multipotent HSPCs to give rise to low frequencies of non–T-lineage cells.14 These cells, even though representing only a very small proportion of thymocytes, may play an important role especially in the allogeneic setting by contributing to thymic negative selection resulting in donor tolerance.
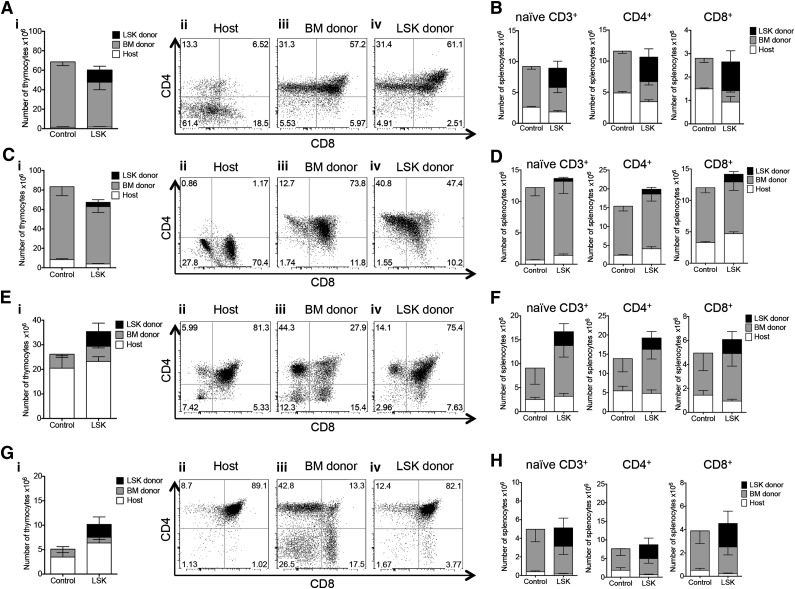
Figure 3.
Intrathymically injected HSPCs contribute to T-cell reconstitution after BMT. (A) Lethally irradiated C57BL/6 recipients were transplanted with C57BL/6.CD45.1+ Lin− BM cells and received 3000 C57BL/6.Thy1.1+ luciferase-expressing LSK cells via ITI 2 hours after irradiation (LSK group). Control mice received Lin− BM only and phosphate-buffered saline (PBS) via ITI 2 hours after irradiation. Thymuses were harvested on day 60 after BMT and analyzed for BM donor and LSK donor origin and CD4 and CD8 DN, DP, SP populations. (i) Mean and SEM of total thymocyte counts are presented (n = 5). (ii-iv) Representative plots of 1 of 5 LSK group samples. Combined data from 2 independent experiments are presented. (B) Animals were treated as described in panel A. Splenocytes were harvested on day 60 after BMT and analyzed for splenic T-cell populations of donor and host origin. Mean and SEM are presented (n = 5). Combined data from 2 independent experiments are presented. (C) Animals were treated as described in panel A. Thymuses were harvested 6 months after BMT and analyzed for BM donor and LSK donor origin and CD4 and CD8 DP, SP, and DN populations. (i) Mean and SEM of total thymocyte counts are presented (n = 4-5). (ii-iv) Representative plots of 1 of 4 LSK group samples. (D) Animals were treated as described in panel A. Splenocytes were harvested 6 months after BMT and analyzed for splenic T-cell populations of donor and host origin. Mean and SEM are presented (n = 4-5). (E) Lethally irradiated BALB/c recipients were transplanted with C57BL/6 TCD BM cells and received 5,000 C57BL/6.CD45.1+ LSK cells via ITI 2 hours after irradiation (LSK group). Control mice received TCD BM only and PBS via ITI 2 hours after irradiation. Thymuses were harvested on day 60 after BMT and analyzed for BM donor and LSK donor origin and CD4 and CD8 DP, SP, and DN populations; 1 of 3 independent experiments is presented. (i) Mean and SEM of total thymocyte counts are presented (n = 4). (ii-iv) Representative plots of 1 of 4 LSK group samples. (F) Animals were treated as described in panel E. Splenocytes were harvested on day 60 after BMT and analyzed for splenic T-cell populations of donor and host origin. Mean and SEM are presented (n = 4). (G) Animals were treated as described in panel E. Thymuses were harvested 6 months after BMT and analyzed for BM donor and LSK donor origin and CD4 and CD8 DP, SP, and DN populations. (i) Mean and SEM of total thymocyte counts are presented (n = 4). (ii-iv) representative plots of 1 of 4 LSK group samples. (H) Animals were treated as described in panel E. Splenocytes were harvested 6 months after BMT and analyzed for splenic T-cell populations of donor and host origin. Mean and SEM are presented (n = 4).
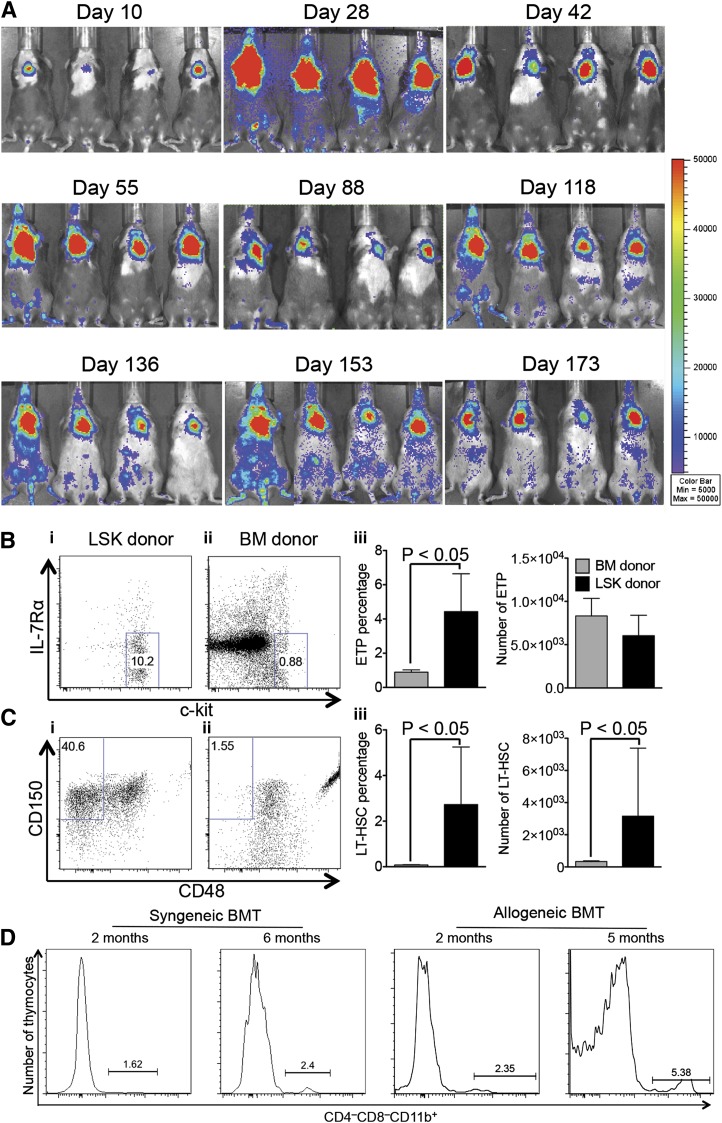
Figure 4.
ITI of multipotent HSPCs results in long-lasting generation of donor T cells. (A) Lethally irradiated C57BL/6 recipients were transplanted with C57BL/6.CD45.1+ Lin− BM cells and received 3000 C57BL/6.Thy1.1+ luciferase-expressing LSK cells via ITI 2 hours after irradiation. The whole-body distribution of LSK-derived cells at the indicated time points after BMT was monitored using in vivo BLI. Pseudocolor images superimposed on conventional photographs are shown. A total of 4 representative animals of 10 are presented. (B) Animals were treated as described in panel A. Thymuses were harvested 6 months after BMT and analyzed for early T-cell progenitor populations (CD25− LSK cells were resolved into subpopulations based on c-kit and IL-7Rα expression) gated on cells of BM donor and LSK donor origin. (i-ii) Representative plots of 1 of 4 LSK group samples are presented. (iii) Mean and SEM are presented (n = 4). (C) Animals were treated as described in panel A. Thymuses were harvested 6 months after BMT and analyzed for long-term HSC populations of BM donor and LSK donor origin (LSK cells were resolved into subpopulations based on CD48 and CD150 expression). (i-ii) Representative plots of 1 of 4 LSK group samples are presented. (iii) Mean and SEM are presented (n = 4). (D) Syngeneic BMT: C57BL/6 recipients were transplanted as described in panel A. Allogeneic BMT: lethally irradiated BALB/c recipients were transplanted with C57BL/6 TCD BM cells and received 5000 C57BL/6.CD45.1+ LSK cells via ITI 2 hours after irradiation. Thymuses were harvested 2 months and 6 months after BMT and analyzed for CD4−CD8−CD11b+ populations of LSK donor origin. Representative plots of 2 to 5 LSK group samples are presented.
ITI of drugs can improve thymopoiesis of aged mice
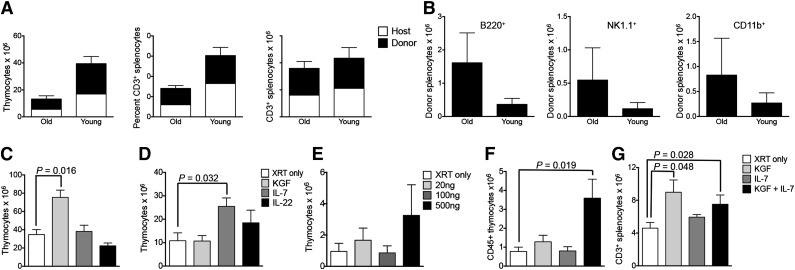
One potential limitation of any immunotherapeutic strategy based on ITI is the difficult visualization of the thymus with increasing age of the recipient. Involution of the human thymus involves replacement of thymic epithelial cells by adipocytes,15 therefore differentiating between bioactive thymic tissue and adipose tissue may be challenging in aged recipients. Nevertheless, it can often still be accomplished by an experienced radiologist.16 In aging mice, the thymus significantly decreases in size but fatty infiltration does not occur. We found that ultrasound imaging allows for accurate assessment of the thymic anatomy in aged mice, and we were able to perform ultrasound-guided free-hand ITIs in up to 2-year-old mice. We established rejuvenated thymopoiesis of sublethally irradiated aged mice by ITI of syngeneic young HSPCs (Figure 5A), although overall thymic cellularity was not increased compared with irradiated controls. This was similar to our observation in young mice (data not shown). We also found a higher proportion of non–T-lineage progeny of intrathymically injected HSPCs in aged compared with young recipients (Figure 5B), reflective of the degenerated thymic microenvironment in aged mice. KGF has been shown to improve thymopoiesis of aged mice through its effects on thymic epithelial cells.17 We therefore assessed if a single ITI of 2 μg of KGF would have a similar effect as the traditional approach (3 doses of 125 μg subcutaneously). We found that our regimen was indeed efficacious in improving thymopoiesis of aged mice (Figure 5C), in contrast to ITI of IL-7 or IL-22, a cytokine that plays an important role in thymic epithelial cell regeneration in response to injury.18 The number of splenic T cells was similar in all groups on day 30 after injection (data not shown). IL-7 has been used in the posttransplant period to improve T-cell reconstitution in both preclinical and clinical studies.19 It is essential for thymic T-cell development as well as survival of peripheral T cells, and IL-7 signaling is known to induce upregulation of antiapoptotic genes in thymocytes and T cells.20 We therefore hypothesized that intrathymically injected IL-7 may increase radioresistance of thymocytes. ITI of 500 ng of IL-7 (but not of KGF or IL-22) prior to radiation exposure indeed improved overall thymic recovery from radiation-induced injury (Figure 5D). Splenic T-cell numbers were not yet affected by day 30 after irradiation (data not shown). Two recent studies revealed that high levels of IL-7 could negatively affect thymopoiesis.21,22 To elucidate a possible dose-dependent differential effect of intrathymically injected IL-7, we first analyzed T-cell generation in response to ITI of low to high doses of IL-7, 1 day prior to radiation in young mice. We found that whereas high-dose IL-7 (500 ng) decreased the percentage of double-negative (DN) 3 thymocytes compared with injection of low-dose IL-7 (20 ng) (supplemental Figure 5), only high-dose IL-7 resulted in increased total thymopoiesis. Moreover, intrathymically injected IL-7 did not alter the distribution of thymic DP or single-positive (SP) populations and did not differentially affect splenic T-cell subsets (data not shown). Next, we repeated the same experiment in aged mice (Figure 5E). Although the benefit of preirradiation IL-7 was not as clear as in young mice, high-dose IL-7 again did not appear to have any negative effect on thymopoiesis. Overall, the most efficacious IL-7 dose for radioprotection of the thymus was therefore 500 ng. Based on our findings in aged as well as in irradiated recipients, we decided to test if combination treatment with KGF and IL-7 had synergistic effects on thymic recovery of aged recipients from radiation-induced injury. Aged mice were conditioned with a single ITI of KGF to increase thymic cellularity. Four weeks later, this was followed by a single injection of IL-7 to confer radioprotection, and all animals were exposed to sublethal TBI 24 hours later. We found that in contrast to our observations in young mice, only the combination of KGF and IL-7 (but not IL-7 alone) resulted in increased thymocyte numbers (mainly due to the effect on CD4 and CD8 DP thymocytes) (Figure 5F and data not shown) as well as increased thymic epithelial cell numbers (supplemental Figure 6A) 4 weeks after irradiation. Notably, KGF alone was sufficient to enhance the generation of mature T cells (Figure 5G and supplemental Figure 6B) 4 weeks after irradiation. Our data suggest that innovative uses of IL-7 and KGF can contribute to further optimize the efficacy of thymus-based cell therapy.
Figure 5.
ITI of cells or drugs can rejuvenate and enhance thymopoiesis of aged recipients. (A) Eleven-month-old (old) or 5-week-old (young) C57BL/6 recipients received 6000 C57BL/6.CD45.1+ LSK cells via ITI 2 hours after sublethal TBI. Thymuses and spleens were harvested on day 60 after radiation and analyzed for donor and host origin and CD3+ populations. Mean and SEM of combined data from 2 independent experiments are presented (n = 5). (B) Old and young C57BL/6 recipients received ITIs with syngeneic LSK cells as described in panel A. Splenocytes were analyzed for B cells, natural killer (NK) cells, and myeloid cells of donor origin on day 60 after radiation. Mean and SEM of combined data from 2 independent experiments are presented (n = 5). (C) Twelve-month-old C57BL/6 mice received 2.5 μg KGF or 500 ng IL-7 or 500 ng IL-22 via ITI. Age-matched control mice received no injection. No irradiation was given to any of the recipients. Thymuses were harvested on day 30 after injection and analyzed for total thymocyte numbers. Mean and SEM of 1 of 3 independent experiments are presented (n = 4). (D) Six-week-old C57BL/6 mice received 2.5 μg KGF or 500 ng IL-7 or 500 ng IL-22 via ITI 24 hours prior to sublethal TBI. Age-matched control mice received no injection. Thymuses were harvested on day 30 after irradiation and analyzed for total thymocyte numbers. Mean and SEM of 1 of 2 independent experiments are presented (n = 5). (E) Nine-month-old BALB/c mice were intrathymically injected with either PBS or 3 different doses of IL-7 cytokine: 20 ng, 100 ng, or 500 ng. Sublethal irradiation was performed on day 1 postinjection. Thymuses were harvested on day 30. Mean and SEM are presented (n = 4-5). (F) Nine-month-old BALB/c mice received a sublethal dose of TBI and a single ITI with either 2.0 μg of KGF on day −28 or 500 ng of IL-7 on day −1 or both. Age-matched control mice received no injection. Thymuses were harvested on day 30 after irradiation and analyzed for total CD45+ thymocyte numbers. Mean and SEM of 1 of 2 independent experiments are presented (n = 4-6). (G) Nine-month-old BALB/c mice were treated as described in panel F. Splenocytes were harvested on day 30 after irradiation and analyzed for total CD3+ T cells. Mean and SEM of 1 of 2 independent experiments are presented (n = 4-6). XRT, radiation therapy.
ITI HSPCs expressing a transgenic TCR facilitates generation of antigen-specific T cells
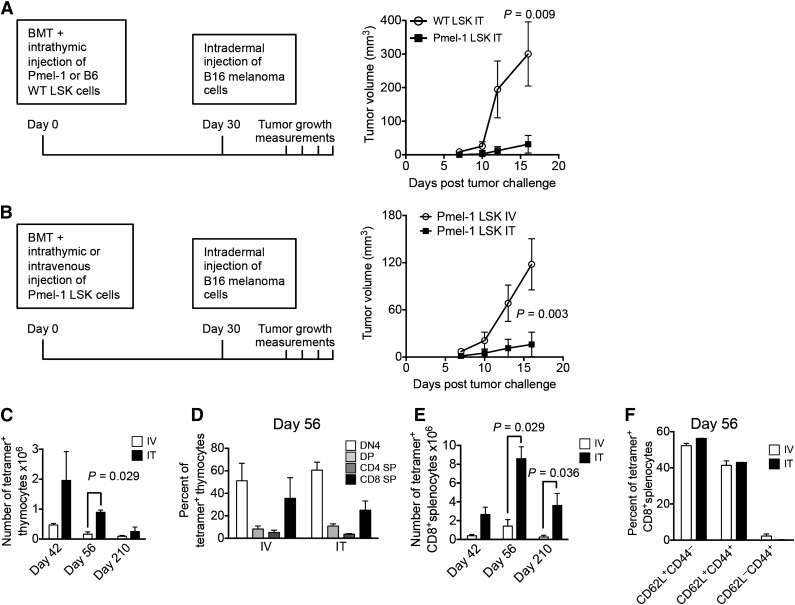
Based on our above-described findings, we hypothesized that ITI of HSPCs can enable the establishment of long-lasting antigen-specific T-cell immunity. We tested our theory in a mouse model of melanoma where B16 melanoma cells were administered by intradermal injection 4 weeks following syngeneic BMT. We first compared antitumor activity displayed by syngeneic BMT recipients that were intrathymically injected 2 hours after BMT with either wild-type (WT) or Pmel-1 syngeneic HSPCs. HSPCs from Pmel-1 mice give rise to T cells expressing a TCR specific for the melanoma-associated peptide Gp100.7,23 As expected, ITI of Pmel-1 HSPCs significantly decreased tumor growth compared with ITI of WT LSK cells (Figure 6A). Moreover, when injecting Pmel-1 HSPCs intravenously versus intrathymically, we found that ITI was associated with significantly improved antitumor activity resulting in a tumor rejection rate of 88% (data not shown), in contrast to animals receiving the same number of Pmel-1 HSPCs intravenously who all developed an intradermal tumor (Figure 6B). Analysis of the kinetics of antigen-specific T-cell reconstitution (up to 7 months follow-up) revealed that intrathymically injected recipients generated significantly more tetramer+ thymocytes (Figure 6C-D; 80% to 96% of DN cells were at the DN4 stage) and 4 times more tetramer+ antigen-specific CD8+ peripheral T cells (Figure 6E). Because Gp100 is a major histocompatibility complex class I restricted antigen, we did not observe any CD4+ tetramer+ peripheral T cells. T cells derived from Pmel-1 HSPCs (both following intravenous injection and ITI) predominantly displayed a naive and central memory phenotype (Figure 6F). This data set, even though not representing a therapeutic tumor model, underscores the potential of our approach for tumor immunosurveillance and targeted immunotherapy.
Figure 6.
ITI of HSPCs from Pmel-1 mice inhibits intradermal growth of melanoma cells. (A) Lethally irradiated C57BL/6 recipients were transplanted with C57BL/6 Lin− BM cells and received 10 000 C57BL/6.Pmel-1 or WT C57BL/6 LSK cells via ITI 2 hours after irradiation. B16 melanoma cells were injected intradermally on day 30 after BMT and tumor volumes were measured manually at days 7, 10, 12, and 16. Mean and SEM are presented (n = 5-6). (B) Lethally irradiated C57BL/6 recipients were transplanted with C57BL/6 TCD BM cells and received 10 000 C57BL/6.Pmel-1 LSK cells intravenously or via ITI 2 hours after irradiation. B16 melanoma cells were injected intradermally on day 30 after BMT and the tumor volume was measured manually at days 7, 10, 13, and 16. Mean and SEM of 1 of 2 independent experiments are presented (n = 8). (C) Animals were transplanted as described in panel B but did not receive tumor cell injections. Thymuses were harvested on days 42, 56, and 210 after BMT and analyzed for tetramer-positive total thymocyte numbers. Mean and SEM of 2 independent experiments are presented (n = 4). (D) Animals were transplanted as described in panel C. Thymuses were harvested on day 56 after BMT and analyzed for tetramer-positive thymocyte subsets. Mean and SEM of 2 independent experiments are presented (n = 4). (E) Animals were transplanted as described in panel C. Spleens were harvested on days 42, 56, and 210 after BMT and analyzed for tetramer-positive CD8+ T cells. Mean and SEM of 2 independent experiments are presented (n = 4). (F) Animals were transplanted as described in panel C. Spleens were harvested on day 56 after BMT and analyzed for tetramer-positive CD8+ T-cell subsets. Mean and SEM of 2 independent experiments are presented (n = 4). IT, intrathymic; IV, intravenous.
Discussion
The goal of this study was to explore innovative strategies to improve the quantity (T-cell numbers) and more importantly the quality (desired antigen specificity) of thymic output in clinically relevant models of thymic insufficiency. ITI allows the manipulation of thymic function; we therefore attempted to develop an improved method for ITI that could facilitate the implementation of novel immunotherapeutic approaches. We were able to accomplish this goal by establishing a method for image-guided free-hand ITI, a technique that is minimally invasive and timesaving, thus facilitating large-scale experiments. Moreover, this method is clinically applicable.
Previous preclinical cell-therapy studies using ITI were performed in recipients with genetic T-cell deficiencies, requiring no thymic conditioning when administering syngeneic BM cells12 and chemotherapy when administering allogeneic BM cells.11 We were able to demonstrate that an immunocompetent recipient can easily be conditioned for ITI by using sublethal TBI or even less toxic thymic irradiation. Moreover, instead of using lineage-marker–negative BM, we established purified LSK cells, the equivalent of human CD34+ HSPCs, as the cell source for this immunotherapy. It is important to note that the cell doses used in this study are likely to be clinically feasible. In most experiments, we injected only a small fraction of the total number of LSK cells that can routinely be isolated from a single donor. ITI of syngeneic HSPCs was associated with virtually lifelong intrathymic persistence (15 months follow-up, equivalent to 50 human years) and continuous T-cell generation. Importantly, we were able to identify progeny of intrathymically injected HSPCs resembling long-term HSCs. Our phenotypic findings combined with the observed long-term intrathymic persistence are suggestive of self-renewal.24,25 Being able to exploit this phenomenon for lifelong immunosurveillance of cancer or life-threatening viral infections, or to promote elimination of minimal residual disease, would be especially beneficial for cancer patients who are at high risk for malignant relapse or recurrence of a latent infection. With this in mind, we were indeed able to demonstrate feasibility and efficacy of antigen-specific T-cell immunity established by ITI of multipotent HSPCs expressing a transgenic TCR. Finally, we were able to develop strategies to overcome critical challenges and limitations of thymus-based approaches, such as thymic damage due to radiation-induced injury or age-related thymic involution. We demonstrate that KGF or IL-7, when administered intrathymically, can be effective with a very low single dose, in contrast to the multidose systemic injection approach traditionally used for these agents. Our study is the first to report radioprotective properties of preirradiation IL-7 administration, a cytokine that is usually not started until several weeks after transplant when used in the BMT setting. Moreover, a single ITI of KGF at a dose that is 150-times lower than the standard cumulative dose used for systemic administration has a sustained positive effect on thymopoiesis of aged mice even in the setting of radiation-induced injury. Taken together, our findings indicate that even an aged thymus can be targeted and may be responsive to interventions designed to optimize the generation of naïve T cells.
In conclusion, our studies introduce a novel method for the establishment of long-lasting T-cell immunity to specific antigens, enabled by a single ITI of TCR transgenic multipotent HSPCs. Importantly, an aged recipient, whose thymus has degenerated over time, can also be successfully treated. To ensure success, pretreatment with a thymic trophic factor such as KGF could be beneficial in order to increase the target size in preparation for injection. Hurdles for the clinical translation of this approach are expected to be minimal because magnetic-bead–selected good manufacturing practices–grade autologous CD34+ HSPCs are routinely obtained in the clinic and US Food and Drug Administration–approved protocols for genetic engineering of human HSPCs have already been established.26 A clinical trial would entail engineering of antigen-specific autologous or allogeneic CD34-selected HSPCs (by introducing a transgenic chimeric antigen receptor,27,28 if desired along with an inducible suicide gene as safety feature29) followed by computed tomography–guided ITI in patients who received suitable thymic conditioning. Our findings therefore identify a rational and clinically relevant strategy to improve T-cell immunity in recipients with differential thymic function.
Supplementary Material
Acknowledgments
We thank Jarrod Dudakov for critical review of the manuscript.
This research was supported by National Institutes of Health award numbers R01-HL069929 from the National Heart, Lung, and Blood Institute (M.R.M.v.d.B.), R01-AI100288 from the National Cancer Institute (M.R.M.v.d.B.), R01-AI080455 from the National Institute of Allergy and Infectious Diseases (M.R.M.v.d.B.), R01-AI101406 from the National Heart, Lung, and Blood Institute (M.R.M.v.d.B.), and 1K08CA160659-01 from the National Cancer Institute (J.L.Z.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project has received funding from the European Union's Seventh Programme for research, technological development and demonstration under grant agreement No 602587. Support was also received from the Radiation Effects Research Foundation (RERF-NIAID) (M.R.M.v.d.B.), the Leukemia Research Foundation (J.L.Z), the MSKCC Center for Molecular Imaging and Nanotechnology (CMINT) (J.L.Z), the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center funded by William H. Goodwin and Alice Goodwin, the Lymphoma Foundation, Alex’s Lemonade Stand, the Geoffrey Beene Cancer Research Center at Memorial Sloan Kettering Cancer Center, and the Susan and Peter Solomon Divisional Genomics Program.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.Z.T. designed and performed experiments, analyzed data, and wrote the paper; R.H.T. performed ultrasound-guided ITIs; Y.S. and O.M.S. performed experiments; E.R.L and F.M.K. provided technical support; M.R.M.v.d.B. provided experimental design, edited the manuscript, and supervised the study; and J.L.Z. designed experiments, edited the paper, and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johannes L. Zakrzewski, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: zakrzewj@mskcc.org.
References
- 1.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117(6):1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zlotoff DA, Zhang SL, De Obaldia ME, et al. Delivery of progenitors to the thymus limits T-lineage reconstitution after bone marrow transplantation. Blood. 2011;118(7):1962–1970. doi: 10.1182/blood-2010-12-324954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008;84(4):915–923. doi: 10.1189/jlb.0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24(5):309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holländer GA, Krenger W, Blazar BR. Emerging strategies to boost thymic function. Curr Opin Pharmacol. 2010;10(4):443–453. doi: 10.1016/j.coph.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao YA, Wagers AJ, Beilhack A, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A. 2004;101(1):221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saran N, Łyszkiewicz M, Pommerencke J, et al. Multiple extrathymic precursors contribute to T-cell development with different kinetics. Blood. 2010;115(6):1137–1144. doi: 10.1182/blood-2009-07-230821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luc S, Luis TC, Boukarabila H, et al. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nat Immunol. 2012;13(4):412–419. doi: 10.1038/ni.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vicente R, Adjali O, Jacquet C, Zimmermann VS, Taylor N. Intrathymic transplantation of bone marrow-derived progenitors provides long-term thymopoiesis. Blood. 2010;115(10):1913–1920. doi: 10.1182/blood-2009-06-229724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Barros SC, Vicente R, Chebli K, Jacquet C, Zimmermann VS, Taylor N. Intrathymic progenitor cell transplantation across histocompatibility barriers results in the persistence of early thymic progenitors and T-cell differentiation. Blood. 2013;121(11):2144–2153. doi: 10.1182/blood-2012-08-447417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adjali O, Vicente RR, Ferrand C, et al. Intrathymic administration of hematopoietic progenitor cells enhances T cell reconstitution in ZAP-70 severe combined immunodeficiency. Proc Natl Acad Sci U S A. 2005;102(38):13586–13591. doi: 10.1073/pnas.0504268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13(1):102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Obaldia ME, Bell JJ, Bhandoola A. Early T-cell progenitors are the major granulocyte precursors in the adult mouse thymus. Blood. 2013;121(1):64–71. doi: 10.1182/blood-2012-08-451773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixit VD. Thymic fatness and approaches to enhance thymopoietic fitness in aging. Curr Opin Immunol. 2010;22(4):521–528. doi: 10.1016/j.coi.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26(1):56–64. doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Min D, Panoskaltsis-Mortari A, Kuro-O M, Holländer GA, Blazar BR, Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109(6):2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudakov JA, Hanash AM, Jenq RR, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336(6077):91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11(5):330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Q, Li WQ, Hofmeister RR, et al. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24(14):6501–6513. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Kassar N, Lucas PJ, Klug DB, et al. A dose effect of IL-7 on thymocyte development. Blood. 2004;104(5):1419–1427. doi: 10.1182/blood-2004-01-0201. [DOI] [PubMed] [Google Scholar]
- 22.El-Kassar N, Flomerfelt FA, Choudhury B, et al. High levels of IL-7 cause dysregulation of thymocyte development. Int Immunol. 2012;24(10):661–671. doi: 10.1093/intimm/dxs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Heijst JW, Ceberio I, Lipuma LB, et al. Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat Med. 2013;19(3):372–377. doi: 10.1038/nm.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins VC, Ruggiero E, Schlenner SM, et al. Thymus-autonomous T cell development in the absence of progenitor import. J Exp Med. 2012;209(8):1409–1417. doi: 10.1084/jem.20120846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peaudecerf L, Lemos S, Galgano A, et al. Thymocytes may persist and differentiate without any input from bone marrow progenitors. J Exp Med. 2012;209(8):1401–1408. doi: 10.1084/jem.20120845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boztug K, Schmidt M, Schwarzer A, et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N Engl J Med. 2010;363(20):1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davila ML, Brentjens R, Wang X, Rivière I, Sadelain M. How do CARs work?: Early insights from recent clinical studies targeting CD19. OncoImmunology. 2012;1(9):1577–1583. doi: 10.4161/onci.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marin V, Cribioli E, Philip B, et al. Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum Gene Ther Methods. 2012;23(6):376–386. doi: 10.1089/hgtb.2012.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.