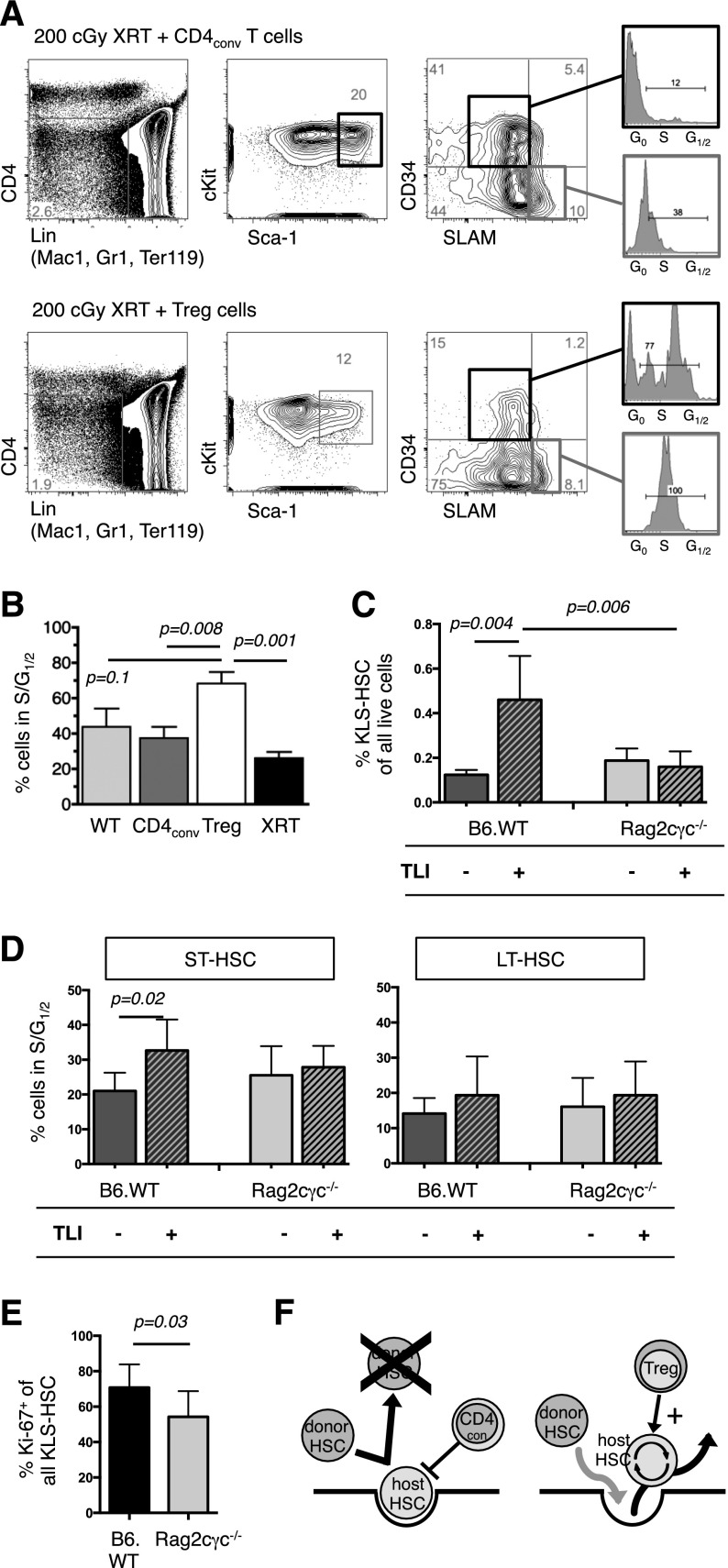
Figure 7.
Tregs influence HSC activity. (A) FACS analysis at day +8 of BM from B6.Rag2γc−/− that had received 200 cGy TBI and IV infusion of 5 × 105 CD4conv (top) or 2 × 105 Treg (bottom). FACS plots of representative animals show the gating strategy to delineate HSC populations, with MPP defined by a CD4−Lin− (Mac1, Gr1, Ter119) cKIT+ Sca-1+ CD34+ SLAM− phenotype and their cell cycle activity. (B) Increased cell cycle activity, as determined by S and G1/2 phases were found in MPP of Treg recipients (N = 3 to 5 per group). (C) BM cells of legs from untreated TLI/ATG-treated B6.WT or B6.Rag2γc−/− mice were analyzed by FACS at 30 hours after the final radiation dose. After TLI/ATG conditioning, B6 WT mice had a significantly higher proportion of KLS-HSC compared with baseline, whereas Rag2γc−/− mice had no detectable increase. (D) In B6.WT, but not in B6.Rag2γc−/− mice S/G1/2 cell cycling activity in ST-HSC was significantly increased following TLI/ATG treatment. Cell cycling activity of LT-HSC was similar in both groups. (E) The increased proliferative activity of the HSC compartment was confirmed by higher Ki-67-expression in B6. WT vs B6.Rag2γc−/− mice at the end of TLI/ATG treatment. (C-E) N = 8 to 11 per experimental group. (F) Hypothesis on T-cell–mediated regulation of HSC activity post-HCT: in the presence of CD4conv cells host HSC decrease cell cycling activity and remain in a quiescent state, which leads to occupation of the niche and failure of donor HSC to replace endogenous host HSC. The presence of Treg increases cell cycling of HSC and their egress into circulation, thereby resulting in open niche space that becomes available for donor HSC in a competitive fashion.