Summary
Neuroectodermal tumours in man, including medulloblastoma, medulloepithelioma, neuroblastoma, esthesioneuroblastoma, primitive neuroectodermal tumour and dysembryoplastic neuroepithelial tumour, typically occur in children and young adults. These tumour types are occasionally observed in juvenile and adult zebrafish (Danio rerio), either as induced tumours in carcinogen-exposed zebrafish or as an incidental finding in zebrafish ≥ 2 years of age. An adult zebrafish submitted for routine histological examination was sent for a second opinion consultation after an uncharacteristic brain mass was identified. Microscopically, the expansile and infiltrative extracortical mass arising from the cerebellum had a diffuse microcystic pattern with solid hypercellular regions occupying 80% of the extrameningeal space and effacing the endomeninx and significantly displacing the metencephalon. The mass was composed of dense sheets of oligodendrocyte-like cells, random neurons and pseudocysts containing ‘floating neurons’ within a scant mucinous matrix. Neoplastic cells demonstrated positive perinuclear and intracytoplasmic expression of S-100. Malignant dysembryoplastic neuroepithelial tumour was diagnosed based upon the histologic features of the brain mass, which were indistinguishable from the human tumour. To our knowledge, this is the first report of a dysembryoplastic neuroepithelial tumour in a zebrafish.
Keywords: zebrafish, dysembryoplastic neuroepithelial tumor, brain, neoplasia
Dysembryoplastic neuroepithelial tumour (DNET) is a benign brain tumour arising from dysplastic brain development of the subpial granular layer (Daumas-Duport et al., 1988; Cabiol et al., 1999; Adesina and Rauch, 2010). The tumour affects children and young adults, ranging in age from 9 to 17 years old at the time of diagnosis (Daumas-Duport 1993; Raymond et al., 1994). Dysembryoplastic neuroepithelial tumour of man has three recognized histological patterns: multinodular, nodular and diffuse, with most of the tumour masses localized to the frontal and temporal lobes and occasional occurrence in the parietal and occipital lobes (Hirose 1999; Honavar et al., 1999). Originally described by Daumas-Duport et al. (1988), DNET is composed of invariant specific glioneuronal elements (oligodendrocyte-like cells [OLCs], astrocytes and neurons) with the OLCs forming the bulk of the tumour mass. Hallmark histoarchitectural features of DNET are microcystic or alveolar patterns (Leung et al., 1994;Hirose 1999), with intracystic ‘floating neurons’ in a mucinous matrix and hypercellular background of OLCs. Cortical dysplasia, mitotic figures, nuclear atypia, calcification and necrosis tend to be variable among the histological types of DNET and in many cases are found inconsistently (Daumas-Duport et al., 1999; Hirose 1999; Honavar et al., 1999).
Naturally-occurring neuroectodermal tumours of the central nervous system (CNS) in teleost fishes are uncommonly reported (Fournie and Overstreet, 1985; Kagan et al. 2010), probably due to a low prevalence among natural fish populations and few opportunities to acquire specimens. In wild-caught and laboratory fishes, such as medaka and zebrafish, both spontaneous and induced CNS neuroectodermal tumours occur (Fournie and Overstreet, 1985; Reimschuessel et al., 1989; White 2004; Feitsma et al., 2008; Ju et al., 2009; Kagan et al., 2010). Neuroectodermal tumours of fish arise during development from pluripotent progenitor cells within subependymal proliferation zones of the telencephalon, tectum and cerebellum, similar to the mammalian subventricular zone (Becker and Becker, 2008). The main difference between neuroectodermal CNS tumours in teleost fishes and those in mammals is that adult fish retain an embryonic plasticity and regenerative capability of neuroepithelial cells that favour development of such CNS tumours (Grandel et al., 2006; Tshering et al., 2008).
An adult wild-type male zebrafish from a sentinel population was reported to have focal cutaneous ulceration on the head, ventrolateral swelling of the body and scoliosis. It was submitted to the Zebrafish International Resource Center, Eugene, Oregon for routine diagnostic screening. The zebrafish was determined to be an older adult based upon both thymic involution and vertebral scoliotic changes in the absence of Pseudoloma neurophilia spinal cord and nerve root infection; although a single isolated Pseudoloma neurophilia pseudocyst containing mature spores was observed within a cranial nerve subjacent to the tumour mass. Vertebral scoliosis is a common indicator of senescence in zebrafish and other teleost fishes due to sarcopenic changes in the dorsal skeletal musculature (Gerhard et al., 2002). The specimen was fixed whole in 10% neutral buffered formalin (total time in fixative unknown), decalcified in CalExII™ solution (10–15% formic acid plus formalin) for 48 h, trimmed parasagittally, processed routinely and embedded in paraffin wax. Tissue sections were stained with haematoxylin and eosin (HE) and a special stains panel including periodic acid–Schiff (PAS), mucicarmine and luxol fast blue (LFB).
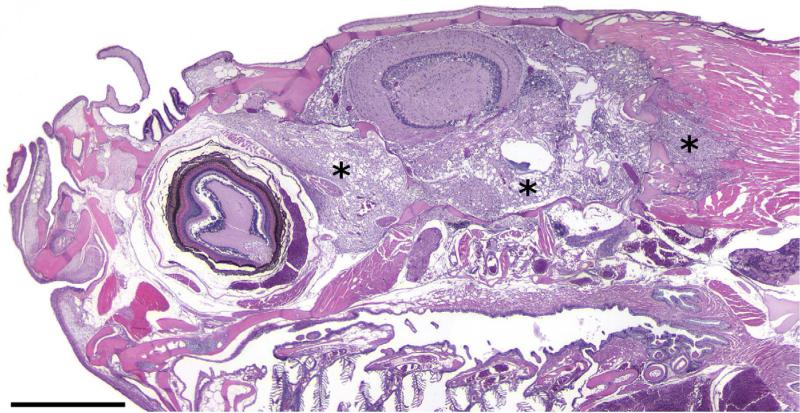
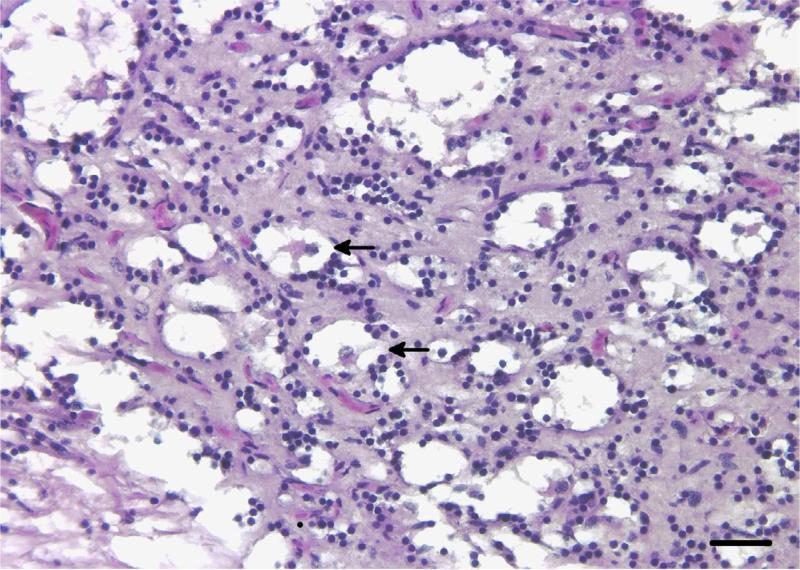
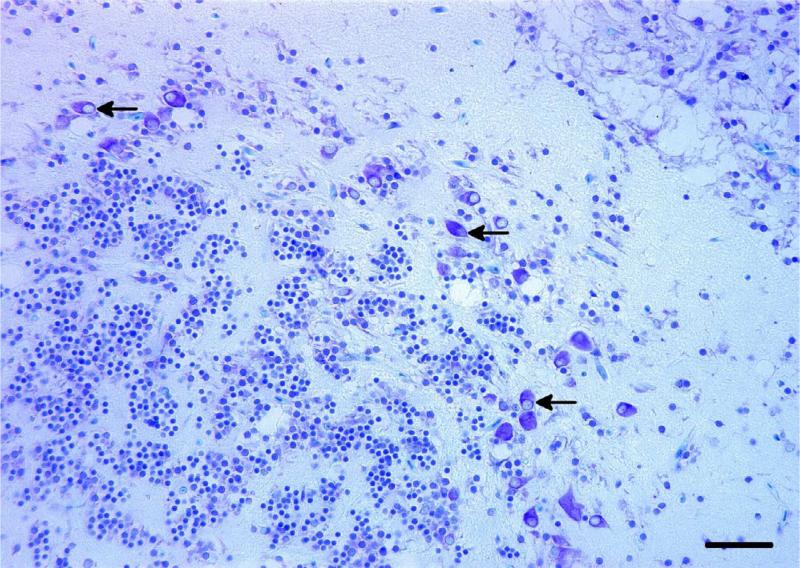
Microscopically, the brain mass revealed a poorly demarcated, expansile and infiltrative extracortical neoplastic mass occupying the endomeningeal space and emanating from a solitary dysplastic cortical focus within the cerebellar external granular cell layer containing dysmorphic neurons and putative ‘balloon’ cells, for which specific diagnostic criteria have been described (Palmini et al., 2004). The brain mass extended into the retrobulbar space adjacent to the choroid rete, around osseous bullae of the inner ears and dorsally along the brain stem and spinal cord. Invasive sheets of tumour cells penetrated through the exoccipital and basioccipital bones of the neurocranium, extending into and dissecting individual skeletal muscle fibre bundles. At low magnification the mass had a diffuse microcystic appearance with a densely cellular background similar in appearance to the brain neuropil (Fig.1). Within the intracystic spaces there were peripheralized and infrequent central ‘floating neurons’ of variable sizes surrounded by a sparsely mucinous matrix (Fig. 2). Solid areas of the mass were composed of hypercellular regions of OLCs with round hyperchromatic nuclei, inconspicuous nucleoli and minimal to inapparent cytoplasm, often with a perinuclear halo or ‘fried egg’ appearance reminiscent of those seen in an oligodendroglioma. Many of the pseudocysts appeared to be lined by variable numbers of these OLCs. Dysmorphic neurons, rare binucleated neurons and putative balloon cells were observed throughout the mass. OLCs occasionally displayed moderate nuclear pleomorphism and karyomegaly. Mitotic figures, perineuronal satellitosis, perivascular aggregates of OLCs, glomeruloid vascular proliferation and necrosis were not seen in any areas of the tumour. The LFB stain highlighted intratumoural neurons and neurons in the focal area of cerebellar cortical dysplasia. Misshapen and abnormally oriented neurons (interpreted as dysmorphic neurons), as well as putative balloon cells, were identified in the area of cortical dysplasia (Fig. 3). Mucicarmine staining revealed lightly positive staining of the intracystic mucinous material when it was present. The PAS stain demonstrated sparse intracystic PAS-positive mucofibrillar material. S-100 immunohistochemical labelling of the tumour cells was inconsistent, with punctate perinuclear and diffuse intracytoplasmic labelling of individual OLCs. Glial cells serving as an internal positive control also had inconsistent labelling.
Fig. 1.
Tumour growth pattern and infiltrative nature is revealed by sheets of neoplastic cells (asterisks) extending through the neurocranium into the retrobulbar space, around the inner ear and dissecting between skeletal muscle fibre bundles. HE. Bar, 1 mm.
Fig. 2.
Microcystic pattern of the tumour growth containing intracystic ‘floating neurons’ embedded in sparse mucinous material (arrows) with a hypercellular background composed of OLCs. HE. Bar, 25 μm.
Fig. 3.
Abnormally oriented and misshapen dysmorphic neurons with eccentric nuclei and putative balloon cells (arrows) within a focus of cerebellar cortical dysplasia adjacent to the tumour. LFB. Bar, 25 μm.
Additional findings included a focal non-ulcerated integumentary defect devoid of scales on the craniodorsal calvarium (most likely the ‘parietal eye’) and a spermatocytic seminoma. A diagnosis of malignant DNET was made because the histomorphology and cellular components of the zebrafish brain tumour resembled the diffuse form of human DNET and closely paralleled the established human diagnostic criteria, including those for the rare malignant version of DNET (Hammond et al., 2000). Features of malignancy in the zebrafish DNET included OLC cellular atypia and an infiltrative growth pattern. Malignant transformation of DNET has only been reported twice in the human medical literature (Hammond et al., 2000; Rushing et al., 2003).
DNET and other glioneuronal tumours arise from pluripotent neuroglia (i.e. oligodendrocyte-like or oligodendroglial cells; Pal et al., 2002) that are retained in zebrafish and other teleost fishes throughout development (Ekstrom et al., 2001). Neuroglia originates from specific proliferation and migratory zones in several brain areas including ependymal and subependymal regions of telencephalic and tectal ventricles. Neuroglia that differentiate into oligodendroglial cells in the zebrafish brain initially appear and mature in the ventral hindbrain (metencephalon) by 4 days post fertilization (dpf) and then extend rostrally to the midbrain (mesencephalon) at 10 dpf (Wullimann and Knipp, 2000; Brosamle and Halpern, 2002; Yoshida and Macklin, 2005). Within cerebellar subdivisions, neuroglia in the valvula and corpus cerebelli continuously migrates to respective granular layers, differentiating into either neurons or oligodendrocytes (Zupanc et al. 2005). Given the anatomical location (the cerebellar external granular layer) the DNET in this zebrafish may have derived from this population of embryonic neuroglia during early development and developed slowly over time. S-100 protein was selected as the immunohistochemical marker to identify intratumoural OLCs based on the work of Hirose et al. (1994), which showed most intratumoural OLCs to be S-100 positive. In human DNETs, OLC S-100 reactivity was reported in the nucleus and cytoplasm, but neurons were weakly reactive or negative (Hirose et al., 1994; Gyure et al., 2000). In the zebrafish brain tumour, patchy S-100 expression was observed in the perinuclear and intracytoplasmic regions of OLCs. Labelling variation of the S-100 zebrafish OLCs may be attributable to using unvalidated mammalian S-100 antibody (1 in 400 dilution; rabbit anti-bovine S-100; DakoCytomation, Dako, Carpinteria, California, USA), as mammalian antibodies produce inconsistent results when applied to fish tissues (Kagan et al. 2010), or because tumour cells were not differentiated enough to allow expression of S-100 protein. The lack of strong staining by either mucicarmine or PAS is most likely due to loss of mucinous material during tissue processing.
Neuroectodermal tumours in fish are caused by heritable genetic defects and exposure of early life stage medaka and zebrafish to environmental carcinogens. Defects in mismatch repair (MMR) of DNA replication errors induced neurofibroma and olfactory neuroblastoma in 6-month to 2-year-old zebrafish mutants with loss-of-function in the major MMR genes (Feitsma et al., 2008). Postembryonic increased expression of fibroblast growth factor 8 (fgf8) in zebrafish insertional mutants has led to the development of neuroblastoma by preventing terminal differentiation of neural precursor cells (Amsterdam et al., 2009). Co-expression of both the hedgehog and AKT pathways has led to the development of glioblastoma and astrocytoma in transgenic zebrafish <3 months of age (Ju et al. 2009). Environmental carcinogens such as the direct-acting nitrosamide N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) has been reported to cause esthesioneuroblastoma in medaka (Spitsbergen et al., 2000b). Polycyclic aromatic hydrocarbon 7, 12-dimethyl-benz[a]anthracene (DMBA) has been shown to cause neuroblastoma in zebrafish (Spitsbergen et al., 2000a).
Based on histomorphology, staining characteristics and S-100 OLC immunohistochemistry (IHC), the brain tumour described herein is, to our knowledge, the first reported case of DNET in a zebrafish. Although its aetiology is uncertain, heritable genetic defects or environmental carcinogens have been shown to cause neuroectodermal tumours in zebrafish.
Acknowledgments
The authors would like to thank K. Fischer, M. Corbus and R. Norred for excellent histotechnical assistance. This work was supported by National Institutes of Health NCRR grants 5R24RR017386-02, 1 T32 RR023917 and P40 RR12546.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesina AM, Rauch RA. Atlas of Pediatric Brain Tumors, Part 5. Springer; New York: 2010. Dysembryoplastic neuroepithelial tumor. pp. 171–180. [Google Scholar]
- Amsterdam A, Lai K, Komisarczuk AZ, Becker TS, Bronson RT, et al. Zebrafish Hagoromo mutants up-regulate fgf8 postembryonically and develop neuroblastoma. Molecular Cancer Research. 2009;7:841–850. doi: 10.1158/1541-7786.MCR-08-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CG, Becker T. Adult zebrafish as a model of successful central nervous system regeneration. Restorative Neurology and Neuroscience. 2008;26:71–80. [PubMed] [Google Scholar]
- Brosamle C, Halpern ME. Characterization of myelination in the developing zebrafish. GLIA. 2002;39:47–57. doi: 10.1002/glia.10088. [DOI] [PubMed] [Google Scholar]
- Cabiol J, Aceba JJ, Isamat F. Dysembryoplastic neuroepithelial tumor. Critical Reviews in Neurosurgery. 1999;9:116–125. doi: 10.1007/s003290050118. [DOI] [PubMed] [Google Scholar]
- Daumas-Duport C. Dysembryoplastic neuroepithelial tumors. Brain Pathology. 1993;3:283–295. doi: 10.1111/j.1750-3639.1993.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Daumas-Duport C, Schiethauer BW, Chodkiewicz JP, Laws ER, Vendrenne C. Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizures. Neurosurgery. 1988;23:545–556. doi: 10.1227/00006123-198811000-00002. [DOI] [PubMed] [Google Scholar]
- Daumas-Duport C, Varlet P, Bacha S, Beuvon F, Cervera-Pierot P, et al. Dysembryoplastic neuroepithelial tumors: non-specific histological forms – a study of 40 cases. Journal of Neuro-oncology. 1999;41:267–280. doi: 10.1023/a:1006193018140. [DOI] [PubMed] [Google Scholar]
- Ekstrom P, Johnsson CM, Ohlin LM. Ventricular proliferation zones in the brain of an adult teleost fish and their relation to neuromeres and migration (secondary matrix) zones. Journal of Comparative Neurology. 2001;436:92–110. [PubMed] [Google Scholar]
- Feitsma H, Kuiper RV, Korving J, Hijman IJ, Cuppen E. Zebrafish with mutations in mismatch repair genes develop neurofibromas and other tumors. Cancer Research. 2008;68:5059–5066. doi: 10.1158/0008-5472.CAN-08-0019. [DOI] [PubMed] [Google Scholar]
- Fournie JW, Overstreet RM. Retinoblastoma in the spring cavefish, Chologaster agassizi Putnam. Journal of Fish Diseases. 1985;8:377–381. [Google Scholar]
- Gerhard GS, Kauffman EJ, Wang X, Stewart R, Moore JL, et al. Life spans and senescent phenotypes in two strains of zebrafish (Danio rerio). Experimental Gerontology. 2002;37:1055–1068. doi: 10.1016/s0531-5565(02)00088-8. [DOI] [PubMed] [Google Scholar]
- Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Developmental Biology. 2006;295:263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Gyure KA, Sandberg GD, Prayson RA, Morrison AL, Armstrong RC, et al. Dysembryoplastic neuroepithelial tumor: an immunohistochemical study with myelin oligodendrocyte glycoprotein. Archives in Pathology and Laboratory Medicine. 2000;124:123–126. doi: 10.5858/2000-124-0123-DNT. [DOI] [PubMed] [Google Scholar]
- Hammond RR, Duggal N, Woulfe JMJ, Girvin JP. Malignant transformation of a dysembryoplastic neuroepithelial tumor. Journal of Neurosurgery. 2000;92:722–725. doi: 10.3171/jns.2000.92.4.0722. [DOI] [PubMed] [Google Scholar]
- Hirose T. Dysembryoplastic neuroepithelial tumor. Neuropathology. 1999;19:233–237. [Google Scholar]
- Hirose T, Scheithauer BW, Beatriz M, Lopes S, VandenBerg SR. Dysembryoplastic neuroepithelial tumor (DNT): an immunohistochemical and ultrastructural study. Journal of Neuropathology and Experimental Neurology. 1994;53:184–195. doi: 10.1097/00005072-199403000-00010. [DOI] [PubMed] [Google Scholar]
- Honavar M, Janota I, Polkey CE. Histological heterogeneity of dysembryoplastic neuroepithelial tumour: identification and differential diagnosis in a series of 74 cases. Histopathology. 1999;34:342–356. doi: 10.1046/j.1365-2559.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Ju B, Spitsbergen J, Eden CJ, Taylor MR, Chen W. Co-activation of hedgehog and AKT pathways promote tumorigenesis in zebrafish. Molecular Cancer. 2009;8:40–46. doi: 10.1186/1476-4598-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan RA, Pinkerton ME, Kinsel MJ. Neuronal embryonal tumors in fish. Veterinary Pathology. 2010;47:553–559. doi: 10.1177/0300985809359600. [DOI] [PubMed] [Google Scholar]
- Leung SY, Gwi E, Ng HK, Yam KY. Dysembryoplastic neuroepithelial tumor. A tumor with small neuronal cells resembling oligodendrogliomas. American Journal of Surgical Pathology. 1994;18:604–614. [PubMed] [Google Scholar]
- Pal L, Shankar SK, Santosh V, Yasha TC. Glioneuronal migration and development disorders: histological and immunohistochemical study with a comment on evolution. Neurology India. 2002;50:444–451. [PubMed] [Google Scholar]
- Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, et al. Terminology and classification of the cortical dysplasias. Neurology. 2004;62(Suppl. 3):S2–S8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- Raymond AA, Halpin SFS, Alsanjari N, Cook MJ, Kitchen ND, et al. Dysembryoplastic neuroepithelial tumour: Features in 16 patients. Brain. 1994;117:461–475. doi: 10.1093/brain/117.3.461. [DOI] [PubMed] [Google Scholar]
- Reimschuessel R, Bennett RO, May EB, Lipsky MM. Retinoblastoma in a porkfish (Anisotremus virginicus, Linnaeus) and a brown bullhead (Ictalurus nebulosus, Lesueur). Journal of Comparative Pathology. 1989;101:215–220. doi: 10.1016/0021-9975(89)90067-4. [DOI] [PubMed] [Google Scholar]
- Rushing EJ, Thompson LD, Mena H. Malignant transformation of a dysembryoplastic neuroepithelial tumor after radiation and chemotherapy. Annals of Diagnostic Pathology. 2003;7:240–244. doi: 10.1016/s1092-9134(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Tsai HW, Reddy A, Miller T, Arbogast D, et al. Neoplasia in zebrafish (Danio rerio) treated with 7,12-dimethylbenz[a]anthracene by two exposure routes at different developmental stages. Toxicologic Pathology. 2000a;28:705–715. doi: 10.1177/019262330002800511. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Tsai HW, Reddy A, Miller T, Arbogast D, et al. Neoplasia in zebrafish (Danio rerio) treated with N-methyl-N’-nitro-N-nitrosoguanidine by three exposure routes at different developmental stages. Toxicologic Pathology. 2000b;28:716–725. doi: 10.1177/019262330002800512. [DOI] [PubMed] [Google Scholar]
- Tshering S, Fimble SM, Mallory DE, Maaswinkel H, Spritzer SD, et al. Ganglion cell regeneration following whole-retina destruction in zebrafish. Developmental Neurobiology. 2008;68:166–181. doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MR. Primitive neuroectodermal neoplasia in Coho salmon, Oncorhynchus kisutch. Veterinary Pathology. 2004;41:72–74. doi: 10.1354/vp.41-1-72. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Knipp S. Proliferation pattern changes in the zebrafish brain from embryonic through early postembryonic stages. Anatomy and Embryology. 2000;202:385–400. doi: 10.1007/s004290000115. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Macklin WB. Oligodendrocyte development and myelination in GFP-transgenic zebrafish. Journal of Neuroscience Research. 2005;81:1–8. doi: 10.1002/jnr.20516. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH, Hinsch K, Gage FH. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. Journal of Comparative Neurology. 2005;488:290–319. doi: 10.1002/cne.20571. [DOI] [PubMed] [Google Scholar]