Abstract
Aim:
To discover and optimize a series of novel PTP1B inhibitors containing a thiazolidinone-substituted biphenyl scaffold and to further evaluate the inhibitory effects of these compounds in vitro and in vivo.
Methods:
A total of 36 thiazolidinone substituted biphenyl scaffold derivatives were prepared. An in vitro biological evaluation was done by Enzyme-based assay. The in vivo efficacy of 7Fb as an antihyperglycemic agent was evaluated in a BKS db/db diabetic mouse model with a dose of 50 mg·kg-1·d-1 for 4 weeks.
Results:
The in vitro biological evaluation showed that compounds 7Fb and 7Fc could increase the insulin-induced tyrosine phosphorylation of IRβ in CHO/hIR cells. In in vivo experiments, compound 7Fb significantly lowered the postprandial blood glucose, from 29.4±1.2 mmol/L with the vehicle to 24.7±0.6 mmol/L (P<0.01), and the fasting blood glucose from 27.3±1.5 mmol/L with the vehicle to 23.6±1.2 mmol/L (P<0.05).
Conclusion:
A novel series of compounds were discovered to be PTP1B inhibitors. Among them, compound 7Fb significantly lowered the postprandial and fasting glucose levels, and the blood glucose level declined more rapidly than in metformin-treated mice. Thus, 7Fb may be a potential lead compound for developing new agents for the treatment of type II diabetes.
Keywords: protein tyrosine phosphatases (PTPs), diabetes, antihyperglycemic activity, drug screening
Introduction
The protein tyrosine phosphatases (PTPs) constitute a family of closely related key regulatory enzymes that dephosphorylate phosphotyrosine residues in their protein substrates. They provide a necessary biological counterpart to protein kinases in signal transduction pathways and play an important role in the regulation of many cellular processes, including cell growth and differentiation, metabolism, cell migration, the immune response, cell apoptosis and bone development1, 2, 3, 4, 5, 6. Malfunctions in PTP activity lead to aberrant tyrosine phosphorylation and are linked to various diseases, such as diabetes, obesity, cancer, inflammation and neurodegenerative diseases7, 8, 9, 10. Therefore, the development of therapeutically promising potent PTP inhibitors is of great importance.
Protein tyrosine phosphatase-1B (PTP1B) is an intracellular PTP that is implicated as a key negative regulator of the insulin and leptin signaling pathways11, 12, 13. It acts by dephosphorylating specific phosphotyrosine (pTyr) residues on the insulin receptor and on insulin receptor substrate proteins7, 11, 14, 15, 16. Two landmark papers reported that PTP1B deficient mice are more sensitive to insulin, have improved glycemic control, and are resistant to diet induced obesity17, 18. Furthermore, treatment of diabetic mice with PTP1B antisense oligonucleotides reduced the expression level of this enzyme and subsequently normalized blood glucose levels and improved insulin sensitivity19, 20. A PTP1B inhibitor may provide a novel strategy for the treatment of type II diabetes and obesity. Recent studies have shown that PTP1B also plays a role in tumorigenesis10, 21. As a result, PTP1B inhibitors represent attractive pharmaceutical agents for treating type II diabetes, obesity, and cancer. Thus, over the past decade, numerous PTP1B inhibitors have been developed to be used as drug candidates22, 23, 24, 25. Most of the reported compounds have exhibited excellent potency (at nanomolar concentrations) in in vitro studies, but the low cell permeability and poor bioavailability of these compounds have limited their application for the development of effective drugs26, 27, 28. Therefore, PTP1B inhibitors still represent a challenge for medicinal chemists.
Compounds of the thiazolidinedione (TZD) class have aroused considerable interest as antihyperglycemic compounds and aldose reductase inhibitors29, 30, 31. Some of these compounds (such as pioglitazone and rosiglitazone) are insulin-sensitizing agents acting as peroxisome proliferator-activated receptor γ (PPARγ) agonists30, and they have been shown to be effective in treating type II diabetes in clinical situation. In addition, some 2,4-TZDs have proved to be PTP1B inhibitors32.
In our previous work, we have reported the discovery of PTP1B inhibitors from our combinatorial library, in which the thiazolidinedione moiety and substituted biphenyl scaffold were found to be effective33. Here we describe our efforts to extend the SAR studies leading to more potent PTP1B inhibitors with antihyperglycemic activity in vivo.
Materials and methods
Chemistry
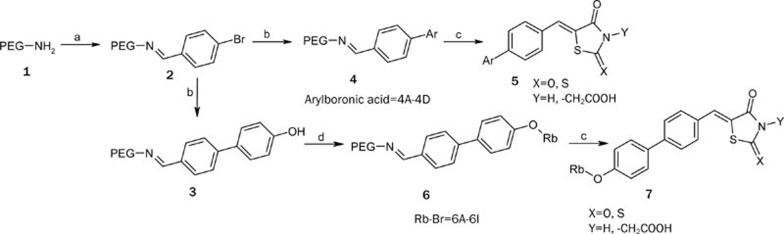
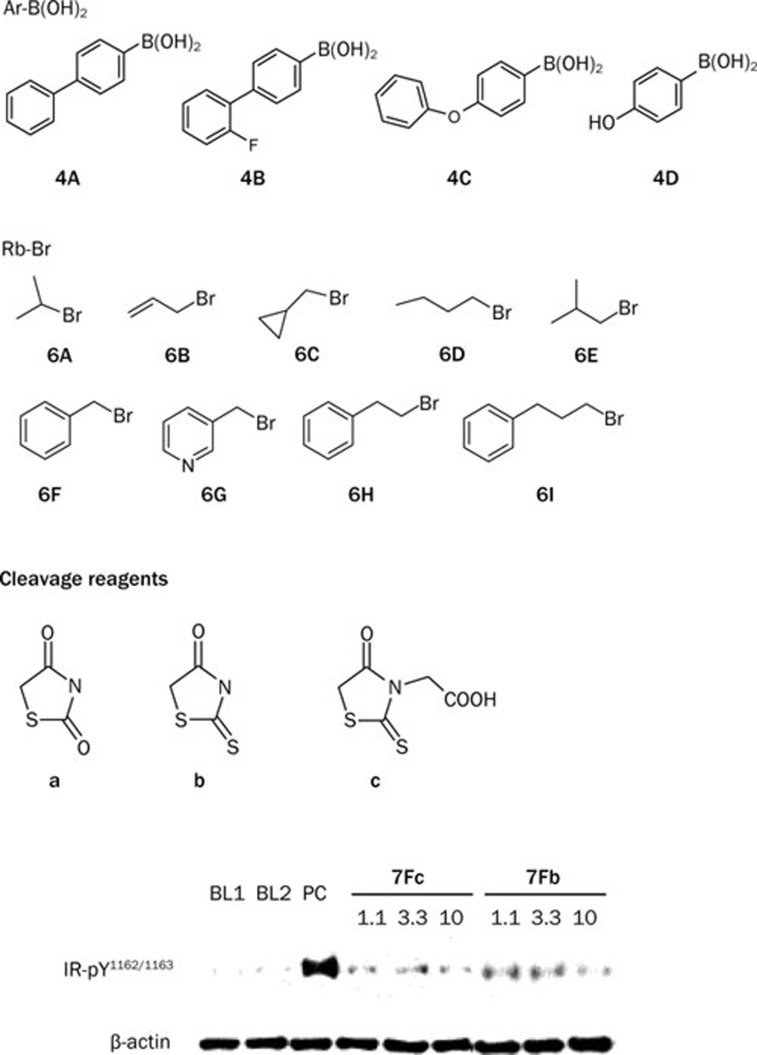
The general method of synthesis for the compounds is depicted in Scheme 1. 4-Bromo benzaldehyde was attached to the amino functionalized PEG support via an imine linkage, and Suzuki coupling was subsequently performed to give polymer 4. Products 5Aa–5Cc were obtained from the cleavage reaction of polymer 4 with different cleavage agents (Scheme 1)33. Since 4'-substituted compounds were identified as more potent PTP1B inhibitors, additional diversity was introduced at the 4'-position of the biphenyl scaffold. Polymer 3 was reacted with halides 6A–6I and then released from the PEG support using the same cleavage strategy to afford products 7Aa–7Ic. This process generally provided the final products in >75 % yield with >85 % purity.
Scheme 1.
Reagents and conditions: a) 4-Bromo benzaldehyde, MeOH, 50 °C, 6 h; b) Ar-B(OH)2 (4A–4D), 1 mol% Pd(OAc)2, K2CO3, MeOH, 80 °C, 8 h; c) Cleavage reagents, AcOH, NaOAc, 120 °C, 12 h; d) Rb-Br (6A–6I), K2CO3, CH3CN, 80 °C, 12 h.
In vitro enzyme assays
Enzyme-based assay of PTP1B
A colorimetric high throughput assay to measure inhibition against PTP1B was performed in 96-well plates. Briefly, the tested compounds were solubilized in DMSO and serially diluted into concentrations for the inhibitory test. The assays were carried out in a final volume of 100 μL containing 50 mmol/L MOPS, pH 6.5, 2 mmol/L pNPP, 30 nmol/L GST-PTP1B, and 2% DMSO, and the catalysis of pNPP was continuously monitored on a SpectraMax 340 microplate reader at 405 nm for 2 min at 30 °C. The IC50 value was calculated from the nonlinear curve fitting of the percent inhibition [inhibition (%)] vs the inhibitor concentration [I] using the following equation: %inhibition=100/{1+(IC50/[I])k}, where k is the Hill coefficient.
Enzyme-based assay of PTP1s
PTPase family members, such as Src homology domain 2 (SH2)-containing tyrosine phosphatase-1 (SHP1), Src homology domain 2 (SH2)-containing tyrosine phosphatase-2 (SHP2), leukocyte antigen-related phosphatase (LAR), CDC25B and PRL-3, were prepared for the selectivity assay of compounds as previously mentioned34. Assays for these PTPases were performed at the optimal pH for each individual enzyme activity. These enzymes and inhibitors were preincubated for 3 min at 4 °C, and the assays were initiated by adding substrates. Assays performed for CDC25B, SHP1 and SHP2, LAR and PRL-3 were done using OMFP as a substrate.
In vivo efficacy study on diabetic BKS db/db mouse
C57BLKS/J–db/db mice were introduced from Jackson Laboratories. At the age of 8 weeks, db/db mice were randomized into the various treatment groups by body weight and random-fed glucose levels. Mice were orally administered once daily with 50 mg/kg per day 7Fb and 150 mg/kg per day metformin. The diabetic and wildtype mice were gavaged with 5% methycellulose (MC) as control group for 4 weeks. The random-fed and fasting blood glucose were tested after 4 weeks treatment. The glucose tolerance test was performed after 6 h fasting and blood glucose were recorded in 0-120 min after 2 g/kg glucose ip injection. Difference between groups was analyzed by Student's t-test. All animal experiments were approved by the Animal Ethics Committee of the Shanghai Institute of Materia Medica.
Results and discussion
Inhibitory activities toward PTP1B
Compounds 5Aa–5Cc and 7Aa–7Ic were evaluated in vitro for their inhibitory activity against PTP1B (Table 1). As illustrated in Table 1, most of these compounds exhibited moderate inhibitory activity, with IC50 values around 10−6mol/L. When comparing 5(A–C)a and 7(A–I)a to 5(A–C)(b, c) and 7(A–I)(b, c), we found that compounds containing a 4-oxothiazolidine-2-thione moiety showed better inhibitory activity against PTP1B. Introduction of an acetic group in the N position of the 4-oxothiazolidine-2-thione moiety made little impact on its activity. Bulky substituents at the 4'-position of the biphenyl scaffold led to favorable bioactivity. Generally, the aryl substituents at the 4'-position provided better inhibition of PTP1B than the alkyl substituent. The length of the linker between the biphenyl scaffold and the aryl group also influenced the inhibitory activity. Benzyl substituents gave the best results in 7Fc and 7Fb, with IC50 values of 0.48±0.07 μmol/L and 0.69±0.07 μmol/L, respectively.
Table 1. In vitro activity against PTP1B.
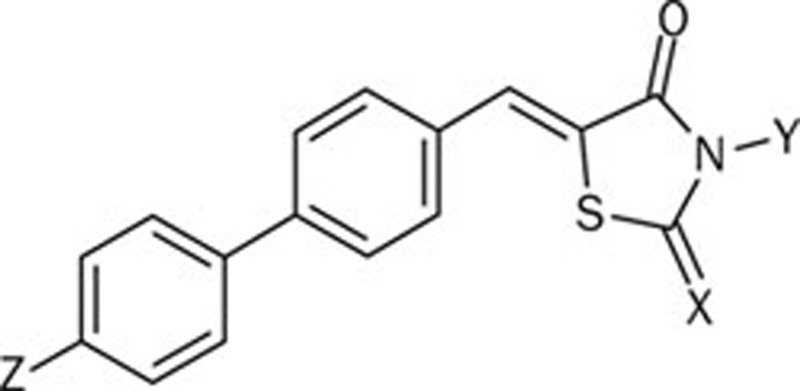
| Compd | Z | X | Y | IC50a (μmol/L) |
|---|---|---|---|---|
| 5Aa | 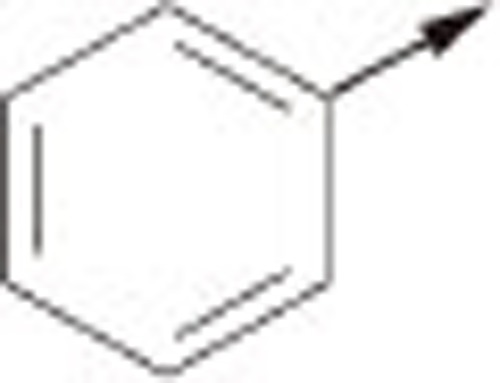 |
O | H | 1.48±0.06 |
| 5Ab | S | H | 1.69±0.24 | |
| 5Ac | S | −CH2COOH | 0.53±0.10 | |
| 5Ba | 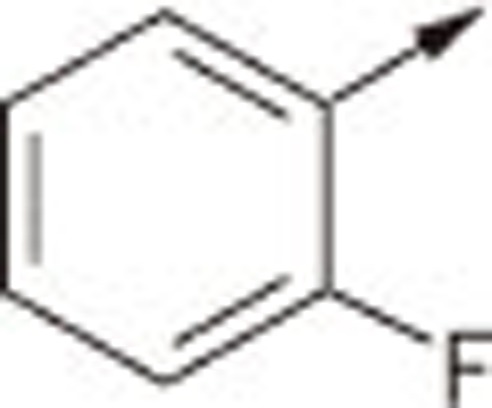 |
O | H | 4.61±0.40 |
| 5Bb | S | H | 2.40±0.15 | |
| 5Bc | S | −CH2COOH | 1.43±0.18 | |
| 5Ca | 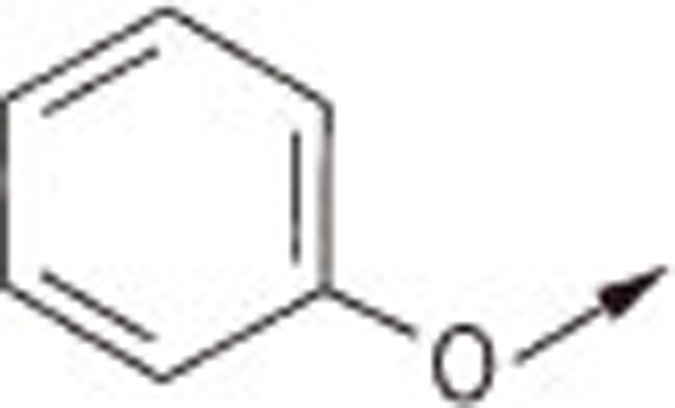 |
O | H | 4.18±0.10 |
| 5Cb | S | H | 0.82±0.03 | |
| 5Cc | S | −CH2COOH | 2.04±0.20 | |
| 7Aa | 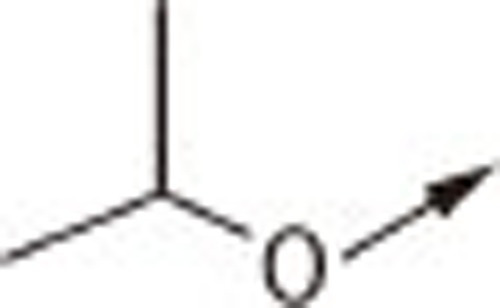 |
O | H | 7.79±0.44 |
| 7Ab | S | H | 4.28±1.04 | |
| 7Ac | S | −CH2COOH | 3.87±0.10 | |
| 7Ba | 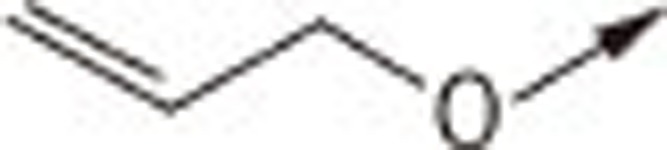 |
O | H | 2.28±0.18 |
| 7Bb | S | H | 1.42±0.20 | |
| 7Bc | S | −CH2COOH | 2.24±0.32 | |
| 7Ca | 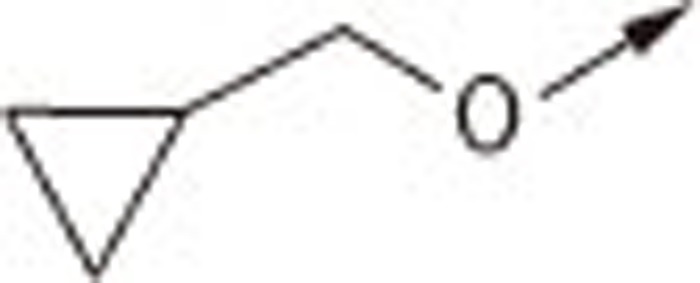 |
O | H | 7.46±0.17 |
| 7Cb | S | H | 1.91±0.00 | |
| 7Cc | S | −CH2COOH | 3.34±0.19 | |
| 7Da |  |
O | H | 12.78±0.48 |
| 7Db | S | H | 2.11±0.19 | |
| 7Dc | S | −CH2COOH | 2.60±0.56 | |
| 7Ea | 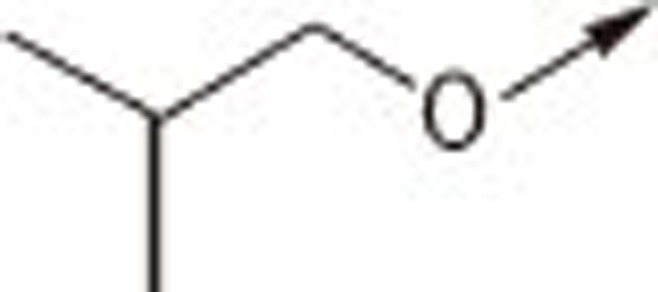 |
O | H | 4.16±0.28 |
| 7Eb | S | H | 1.71±0.14 | |
| 7Ec | S | −CH2COOH | 2.39±0.02 | |
| 7Fa | 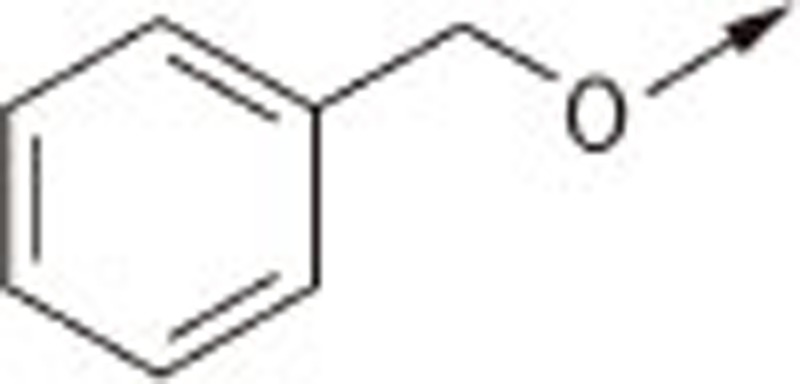 |
O | H | 1.34±0.13 |
| 7Fb | S | H | 0.69±0.07 | |
| 7Fc | S | −CH2COOH | 0.48±0.07 | |
| 7Ga | 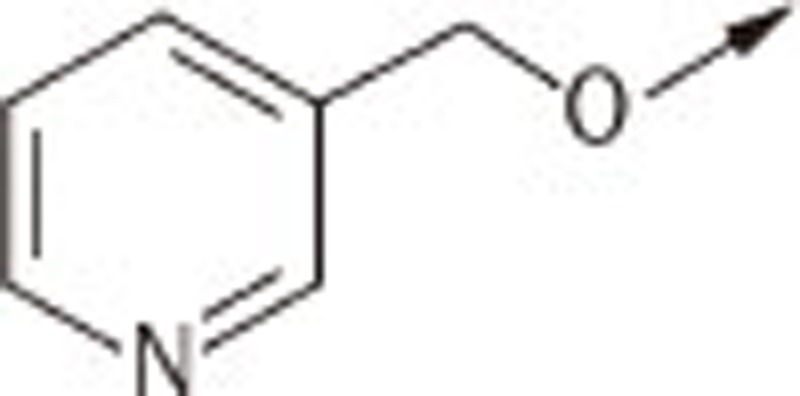 |
O | H | 3.66±0.18 |
| 7Gb | S | H | 1.26±0.10 | |
| 7Gc | S | −CH2COOH | 1.10±0.17 | |
| 7Ha | 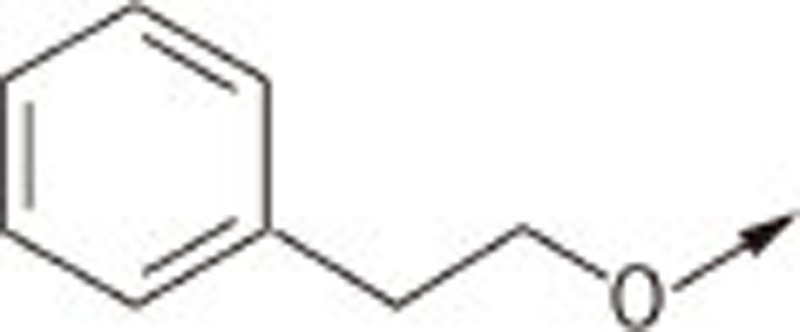 |
O | H | 2.59±0.17 |
| 7Hb | S | H | 1.24±0.14 | |
| 7Hc | S | −CH2COOH | 2.55±0.53 | |
| 7Ia | 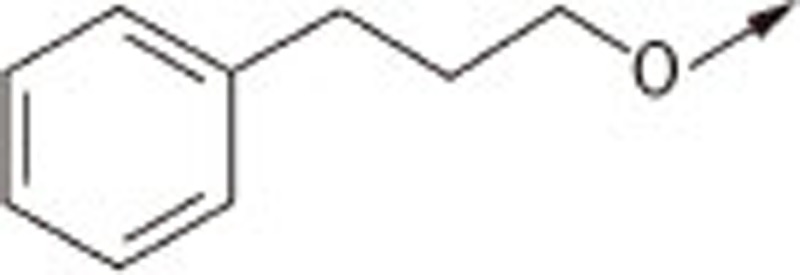 |
O | H | 2.53±0.27 |
| 7Ib | S | H | 1.20±0.12 | |
| 7Ic | S | −CH2COOH | 1.86±0.22 |
Furthermore, 7Fb and 7Fc were screened against a panel of six members of the PTPase family (Table 2). In contrast to the poor selectivity of 7Fc, compound 7Fb exhibited high selectivity against several other therapeutically useful phosphatases (ie, SHP1, SHP2, LAR, etc).
Table 2. Selectivity of 7Fb and 7Fc for a panel of protein phosphatases.
| |
|
|
|
IC50 (μmol/L) |
|
|
|---|---|---|---|---|---|---|
| Compd | PTP1B | LAR | Cdc25B | SHP1 | SHP2 | PRL3 |
| 7Fb | 0.69±0.07 | >50 | 2.22±0.14 | >50 | >50 | 5.34±1.42 |
| 7Fc | 0.48±0.07 | >50 | 0.89±0.03 | 1.90±0.17 | 1.61±0.13 | 2.07±0.50 |
Cellular and in vivo activity of selected compounds
In the next step, we evaluated the two potent inhibitors of PTP1B, 7Fb and 7Fc, in CHO/hIR cells according to our previous method35. CHO/hIR cells were incubated with several concentrations of compounds 7Fb and 7Fc (1.1 μmol/L, 3.3 μmol/L and 10 μmol/L) for 2 h. This was followed by treatment with 10 nmol/L insulin for 10 min (Figure 1). DMSO (0.2%) and orthvanadate (1 mmol/L) were used as negative and positive controls, respectively. The cell lysates were subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and probed with specific anti-pTyr1162/1163 IR antibodies. As shown in Figure 1, both compounds increased the insulin-induced tyrosine phosphorylation of IRβ and compound 7Fb (1.1 μmol/L) boosted IR phosphorylation more potently.
Figure 1.
Effects of 7Fb and 7Fc on tyrosine phosphorylation of IRβ in CHO/hIR cells. The tyrosine phosphorylation level were determined by specific antibody of phosphorylated IR-Tyr1162/1163 with or without treatment, the β-actin represents the sample amount loaded. BL1 and BL2, 0.2% DMSO; PC, 1 mmol/L orthvandate; and the compound centratration unit is μmol/L.
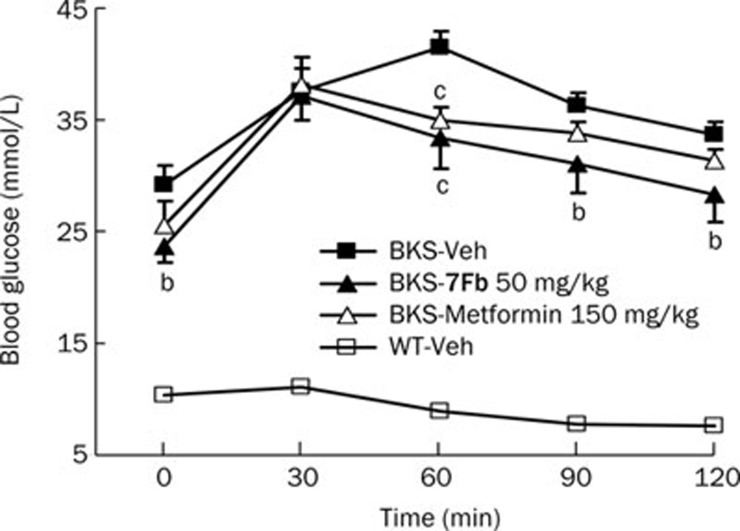
Based on the selective inhibition of PTP1B by 7Fb and its cellular activity of increasing IR phosphorylation, the efficacy study was further investigated in a diabetic mouse model. In vivo efficacy of 7Fb as an antihyperglycemic agent was evaluated in a BKS db/db diabetic mouse model at a dose of 50 mg/kg per day for 4 weeks. Compound 7Fb significantly lowered the postprandial blood glucose from 29.4±1.2 mmol/L with the vehicle to 24.7±0.6 mmol/L (P<0.01) and the fasting blood glucose from 27.3±1.5 mmol/L with the vehicle to 23.6±1.2 mmol/L (P<0.05). The impaired glucose tolerance capacity of the diabetic mice was also significantly improved after prolonged 7Fb treatment, and the area under the curve (AUC) was decreased to 3829.5±208.5 mmol/L·min from 4404.4±100.1 mmol/L·min. The blood glucose level declined more rapidly than in metformin treated mice (150 mg/kg) (Figure 2).
Figure 2.
Glucose tolerance capacity improved by 7Fb. Diabetic BKS db/db mice were treated orally with 7Fb or metformin, the diabetic and wild-type mice were gavaged with 5% methylcellulose (MC) as control group for 4 weeks. The glucose tolerance test (2 g/kg glucose ip) was performed after 6 h fasting and blood glucose level at the above time-points were recorded. Differences between groups were analyzed by Student's t-test. bP<0.05, cP<0.01 vs BKS-Veh.
Taken together, the cellular effect of PTP1B inhibition on the insulin receptor critical tyrosine phosphorylation and the in vivo efficacy of 7Fb in improving the glucose tolerance capacity and blood glucose suggested that PTP1B inhibition was greatly involved in compound 7Fb's bioactivity, but an alternative mode of PPAR activation was not excluded.
Conclusion
In summary, with the methods developed for the synthesis of a biphenyl thiazolidinone library, we have found a series of novel PTP1B inhibitors that exhibited submicromolar potency. Among the compounds, 7Fb was tested in an animal model for its efficacy as an anti-diabetic agent. Compound 7Fb significantly lowered the postprandial and fasting glucose levels and improved the glucose tolerance in the db/db diabetic mice; thus, it may be a potential lead compound for the generation of a therapy for type II diabetes.
Appendix
The reagents (chemicals) were purchased from Lancaster (Morecambe, England), Acros (Geel, Belgium) and Shanghai Chemical Reagent Company (Shanghai, China) and used without further purification. The analytical thin-layer chromatography was done using HSGF 254 (150–200 μm thickness; Yantai Huiyou Company, Yantai, Shandong, China). The 1H NMR (300 MHz or 400 MHz) spectra were recorded on a Varian Mercury-300 or 400 High Performance Digital FT-NMR with TMS as an internal standard, and the 13C NMR (100 MHz) spectra were determined using a Varian Mercury-400 High Performance Digital FT-NMR. Chemical shifts were reported in parts per million (ppm, d) downfield from tetramethylsilane. Proton coupling patterns were described as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), and broad (br). EI-MS and HRMS were performed with a Finnigan MAT 95, EI: 70 eV, R: 10 000. Purity was recorded on a Gilson high-performance liquid chromatography (HPLC) system (306 pump, UV/vis-156 Detector, 215 liquid handle).
General procedures for the preparation of compounds 5Aa–5Cc and 7Aa–7Ic
Compounds 5Aa–5Cc and 7Aa–7Ic were prepared as previously mentioned27.
(Z)-5-((4′-phenylbiphenyl-4-yl)methylene)thiazolidine-2,4-dione (5Aa)
1H NMR (300 MHz, d6-DMSO): δ 7.92 (d, J=8.5 Hz, 2H), 7.87 (d, J=8.5 Hz, 2H), 7.81(m, 3H), 7.73 (m, 4H), 7.49 (m, 2H), 7.38 (m, 1H); 13C NMR (100 MHz, d6-DMSO): 167.845, 167.362, 141.204, 139.933, 139.423, 137.765, 132.181, 131.370, 130.824(×2), 129.079(×2), 127.772, 127.371(×4), 127.312(×2), 126.661(×2), 123.367; EI-MS: m/z 357 (M), 286; HRMS: calculated for C22H15NO2S, 357.0823, found 357.0832; HPLC Purity (retention time): 100% (4.54 min)
(Z)-5-((4′-phenylbiphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (5Ab)
1H NMR (300 MHz, d6-DMSO): δ 7.93 (d, J=8.4 Hz, 2H), 7.87 (d, J=8.4 Hz, 2H), 7.80 (d, J=8.5 Hz, 2H), 7.73 (m, 5H), 7.49 (m, 2H), 7.39 (m, 1H); EI-MS: m/z 373 (M), 286; HPLC Purity (retention time): 98% (4.92 min)
(Z)-2-{4-oxo-5-[(4′-phenylbiphenyl-4-yl)methylene]-2-thioxothiazolidin-3-yl}acetic acid (5Ac)
1H NMR (300 MHz, d6-DMSO): δ 7.92 (m, 3H), 7.89 (d, J=8.5 Hz, 2H), 7.85 (d, J=8.5 Hz, 2H), 7.79 (m, 4H), 7.51 (m, 2H), 7.40 (m, 1H), 4.73 (s, 2H); EI-MS: m/z 431 (M), 306, 286; HPLC Purity (retention time): 96% (3.94 min)
(Z)-5-((4′-2-fluorphenylbiphenyl-4-yl)methylene)thiazolidine-2,4-dione (5Ba)
1H NMR (300 MHz, d6-DMSO): δ 7.96 (d, J=8.5 Hz, 2H), 7.80 (m, 2H), 7.65–7.74 (m, 4H), 7.61 (d, J=8.4 Hz, 2H), 7.50 (m, 2H), 7.43 (m, 1H); 13C NMR (100 MHz, d6-DMSO): 167.722, 167.235, 159.544 (d, JC–F=244.6 Hz), 140.179, 139.806, 134.631, 132.741, 131.338, 131.234, 130.773(×2), 128.742(×3), 128.095, 127.909, 127.476(×2), 123.686, 123.172, 114.431, 114.185; EI-MS: m/z 375 (M), 304; HRMS: calculated for C22H14NO2FS, 375.0729, found 375.0730; HPLC Purity (retention time): 98% (4.37 min)
(Z)-5-((4′-2-fluorphenylbiphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (5Bb)
1H NMR (300 MHz, d6-DMSO): δ 7.97 (d, J=8.5 Hz, 2H), 7.79 (m, 1H), 7.65–7.74 (m, 5H), 7.61 (d, J=8.3 Hz, 2H), 7.51 (m, 2H), 7.43 (m, 1H); EI-MS: m/z 391 (M), 304; HPLC Purity (retention time): 97% (4.76 min)
(Z)-2-(4-oxo-5-((4′-2-fluorphenylbiphenyl-4-yl)methylene)-2-thioxothiazolidin-3-yl)acetic acid (5Bc)
1H NMR (300 MHz, d6-DMSO): δ 7.98 (d, J=8.4 Hz, 2H), 7.92 (s, 1H), 7.79 (m, 1H), 7.68–7.76 (m, 4H), 7.66 (d, J=8.3 Hz, 2H), 7.54 (m, 2H), 7.43 (m, 1H), 4.75 (s, 2H); EI-MS: m/z 449 (M), 304, 226; HPLC Purity (retention time): 98% (3.81 min)
(Z)-5-((4′-phenoxybiphenyl-4-yl)methylene)thiazolidine-2,4-dione (5Ca)
1H NMR (300 MHz, d6-DMSO): δ 7.82 (m, 3H), 7.77 (d, J=8.3 Hz, 2H), 7.67 (d, J=8.3 Hz, 2H), 7.42 (m, 2H), 7.18 (t, J=7.2 Hz, 1H), 7.09 (m, 4H); 13C NMR (100 MHz, d6-DMSO): 167.813, 167.326, 157.205, 156.198, 141.095, 133.802, 131.780, 130.787(×2), 130.209(×2), 128.542(×2), 127.544, 127.116(×2), 123.941, 123.144, 119.150(×2), 118.799(×2); EI-MS: m/z 373 (M), 302, 225; HRMS: calculated for C22H15NO3S, 373.0773, found 373.0771; HPLC Purity (retention time): 100% (4.52 min)
(Z)-5-((4′-phenoxybiphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (5Cb)
1H NMR (300 MHz, d6-DMSO): δ 7.85 (d, J=8.5 Hz, 2H), 7.78 (d, J=8.5 Hz, 2H), 7.69 (m, 3H), 7.43 (m, 2H), 7.19 (t, J=7.5 Hz, 1H), 7.09 (m, 4H); 13C NMR (100 MHz, d6-DMSO): 195.315, 169.257, 157.264, 156.139, 141.350, 133.643, 131.639, 131.247(×2), 130.181(×2), 128.528(×2), 127.581, 127.162(×2), 124.966, 123.937, 119.154(×2), 118.744(×2); EI-MS: m/z 389 (M), 302, 225; HRMS: calculated for C22H15NO2S2, 389.0544, found 389.0539; HPLC Purity (retention time): 100% (4.96 min)
(Z)-2-(4-oxo-5-((4′-phenoxybiphenyl-4-yl)methylene)-2-thioxothiazolidin-3-yl)acetic acid (5Cc)
1H NMR (300 MHz, d6-DMSO): δ 7.89 (s, 1H), 7.86 (d, J=8.6 Hz, 2H), 7.82 (d, J=8.6 Hz, 2H), 7.70 (m, 2H), 7.45 (m, 2H), 7.19 (t, J=7.5 Hz, 1H), 7.09 (m, 4H), 4.75 (s, 2H); EI-MS: m/z 447 (M), 302, 225; HPLC Purity (retention time): 98% (4.16 min)
(Z)-5-((4′-isopropoxybiphenyl-4-yl)methylene)thiazolidine-2,4-dione (7Aa)
1H NMR (300 MHz, d6-DMSO): δ 7.81 (m, 3H), 7.63–7.70 (m, 4H), 7.02 (d, J=8.6 Hz, 2H), 4.68 (m, 1H), 1.29 (d, J=5.7 Hz, 6H); EI-MS: m/z 339 (M), 297, 226; HPLC Purity (retention time): 94% (4.27 min)
(Z)-5-((4′-isopropoxybiphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (7Ab)
1H NMR (300 MHz, d6-DMSO): δ 7.82 (d, J=8.5 Hz, 2H), 7.63–7.70 (m, 5H), 7.02 (d, J=8.7 Hz, 2H), 4.68 (m, 1H), 1.28 (d, J=5.8 Hz, 6H); EI-MS: m/z 355 (M), 313, 226; HPLC Purity (retention time): 99% (4.60 min)
(Z)-2-(5-((4′-isopropoxybiphenyl-4-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (7Ac)
1H NMR (300 MHz, d6-DMSO): δ 7.89 (s, 1H), 7.87(d, J=8.5 Hz, 2H), 7.68–7.75 (m, 4H), 7.03 (d, J=8.7 Hz, 2H), 4.75 (s, 2H), 4.68 (m, 1H), 1.28 (d, J=5.8 Hz, 6H); EI-MS: m/z 413 (M), 371, 226; HPLC Purity (retention time): 98% (3.73 min)
(Z)-5-((4′-(allyloxy)biphenyl-4-yl)methylene)thiazolidine-2,4-dione (7Ba)
1H NMR (300 MHz, d6-DMSO): δ 7.82 (m, 3H), 7.68–7.75 (m, 4H), 7.06 (d, J=8.7 Hz, 2H), 6.05 (m, 1H), 5.42 (dd, J=17.5 Hz, 1.6 Hz, 1H), 5.27 (dd, J=10.5 Hz, 1.6 Hz, 1H), 4.63 (dd, J=5.1 Hz, 1.6 Hz, 2H); EI-MS: m/z 337 (M), 296, 225; HPLC Purity (retention time): 95% (4.35 min)
(Z)-5-([4′-(allyloxy)biphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (7Bb)
1H NMR (300 MHz, d6-DMSO): δ 7.82 (d, J=8.3 Hz, 2H), 7.68 (m, 5H), 7.06 (d, J=8.7 Hz, 2H), 6.05 (m, 1H), 5.41 (dd, J=17.4 Hz, 1.6 Hz, 1H), 5.26 (dd, J=10.6 Hz, 1.6 Hz, 1H), 4.62 (dd, J=5.0 Hz, 1.6 Hz, 2H); EI-MS: m/z 353 (M), 312, 225; HPLC Purity (retention time): 98% (4.72 min)
(Z)-2-(5-((4′-(allyloxy)biphenyl-4-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (7Bc)
1H NMR (300 MHz, d6-DMSO): δ 7.90 (s, 1H), 7.87 (d, J=8.4 Hz, 2H), 7.76 (m, 4H), 7.07 (d, J=8.7 Hz, 2H), 6.06 (m, 1H), 5.42 (dd, J=17.2 Hz, 1.6 Hz, 1H), 5.26 (dd, J=10.4 Hz, 1.6 Hz, 1H), 4.74 (s, 2H), 4.63 (dd, J=5.0 Hz, 1.6 Hz, 2H); EI-MS: m/z 411 (M), 370, 225; HPLC Purity (retention time): 96% (3.80 min)
(Z)-5-((4′-(cyclopropylmethoxy)biphenyl-4-yl)methylene)thiazolidine-2,4-dione (7Ca)
1H NMR (300 MHz, d6-DMSO): δ 7.81 (m, 3H), 7.67–7.74 (m, 4H), 7.03 (d, J=8.6 Hz, 2H), 3.85 (d, J=7.2 Hz, 2H), 1.23 (m, 1H), 0.57 (m, 2H), 0.33 (m, 2H); EI-MS: m/z 351 (M), 297, 226; HPLC Purity (retention time): 94% (4.38 min)
(Z)-5-((4′-(cyclopropylmethoxy)biphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (7Cb)
1H NMR (300 MHz, d6-DMSO): δ 7.81 (d, J=8.5 Hz, 2H), 7.67 (m, 5H), 7.02 (d, J=8.7 Hz, 2H), 3.86 (d, J=7.0 Hz, 2H), 1.23 (m, 1H), 0.57 (m, 2H), 0.33 (m, 2H); EI-MS: m/z 367 (M), 313, 280, 226; HPLC Purity (retention time): 98% (4.79 min)
(Z)-2-(5-((4′-(cyclopropylmethoxy)biphenyl-4-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (7Cc)
1H NMR (300 MHz, d6-DMSO): δ 7.90 (s, 1H), 7.88 (d, J=8.4 Hz, 2H), 7.75 (m, 4H), 7.04 (d, J=8.6 Hz, 2H), 4.75 (s, 2H), 3.86 (d, J=7.1 Hz, 2H), 1.24 (m, 1H), 0.57 (m, 2H), 0.33 (m, 2H); EI-MS: m/z 425 (M), 371, 280, 226; HPLC Purity (retention time): 96% (3.98 min)
(Z)-5-((4′-butoxybiphenyl-4-yl)methylene)thiazolidine-2,4-dione (7Da)
1H NMR (300 MHz, d6-DMSO): δ 7.81 (m, 3H), 7.67–7.74 (m, 4H), 7.03(d, J=8.7 Hz, 2H), 4.02 (t, J=6.7 Hz, 2H), 1.69 (m, 2H), 1.44 (m, 2H), 0.94 (t, J=7.5 Hz, 3H); EI-MS: m/z 353 (M), 297, 226; HPLC Purity (retention time): 96% (4.25 min)
(Z)-5-((4′-butoxybiphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (7Db)
1H NMR (300 MHz, d6-DMSO): δ 7.81 (d, J=8.3 Hz, 2H), 7.67 (m, 5H), 7.03(d, J=8.8 Hz, 2H), 4.01 (t, J=6.6 Hz, 2H), 1.69 (m, 2H), 1.44 (m, 2H), 0.93 (t, J=7.5 Hz, 3H); EI-MS: m/z 369 (M), 282, 226; HPLC Purity (retention time): 94% (4.66 min)
(Z)-2-(5-((4′-butoxybiphenyl-4-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (7Dc)
1H NMR (300 MHz, d6-DMSO): δ 7.89 (s, 1H), 7.87 (d, J=8.5 Hz, 2H), 7.76 (m, 4H), 7.05 (d, J=8.6 Hz, 2H), 4.75 (s, 2H), 4.02 (t, J=6.7 Hz, 2H), 1.69 (m, 2H), 1.45 (m, 2H), 0.94 (t, J=7.5 Hz, 3H); EI-MS: m/z 427 (M), 282, 226; HPLC Purity (retention time): 100% (3.84 min)
(Z)-5-((4′-isobutoxybiphenyl-4-yl)methylene)thiazolidine-2,4-dione (7Ea)
1H NMR (300 MHz, d6-DMSO): δ 7.82 (m, 3H), 7.67–7.74 (m, 4H), 7.03 (d, J=8.7 Hz, 2H), 3.79 (d, J=6.8 Hz, 2H), 2.04 (m, 1H), 0.98 (d, J=6.9 Hz, 6H); EI-MS: m/z 353 (M), 297, 226; HPLC Purity (retention time): 95% (4.31 min)
(Z)-5-((4′-isobutoxybiphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (7Eb)
1H NMR (300 MHz, d6-DMSO): δ 7.81 (d, J=8.7 Hz, 2H), 7.67 (m, 5H), 7.03(d, J=8.7 Hz, 2H), 3.79 (d, J=6.5 Hz, 2H), 2.03 (m, 1H), 0.98 (d, J=6.7 Hz, 6H); EI-MS: m/z 369 (M), 313, 226; HPLC Purity (retention time): 100% (4.70 min)
(Z)-2-(5-((4′-isobutoxybiphenyl-4-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (7Ec)
1H NMR (300 MHz, d6-DMSO): δ 7.89 (s, 1H), 7.87 (d, J=8.7 Hz, 2H), 7.76 (m, 4H), 7.05 (d, J=8.6 Hz, 2H), 4.75 (s, 2H), 3.80 (d, J=6.3 Hz, 2H), 2.03 (m, 1H), 0.99 (d, J=6.5 Hz, 6H); EI-MS: m/z 427 (M), 371, 282, 226; HPLC Purity (retention time): 98% (3.87 min)
(Z)-5-((4′-(benzyloxy)biphenyl-4-yl)methylene)thiazolidine-2,4-dione (7Fa)
1H NMR (300 MHz, d6-DMSO): δ 7.81 (m, 3H), 7.68–7.73 (m, 4H), 7.38–7.48 (m, 5H), 7.13 (m, 2H), 5.17 (s, 2H); 13C NMR (100 MHz, d6-DMSO): 158.931, 158.662, 145.522, 143.978, 136.977, 134.531, 131.457, 131.193, 130.746, 130.218, 129.976, 128.514(×2), 128.423, 128.191, 128.041, 127.927, 127.726(×2), 126.701, 126.196, 115.488, 69.320; EI-MS: m/z 387 (M), 304, 225; HRMS: calculated for C23H17NO3S, 387.0929, found 387.0936; HPLC Purity (retention time): 98% (4.52 min)
(Z)-5-((4′-(benzyloxy)biphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (7Fb)
1H NMR (300 MHz, d6-DMSO): δ 7.82 (d, J=8.5 Hz, 2H), 7.67 (m, 5H), 7.33–7.48 (m, 5H), 7.12 (d, J=8.6 Hz, 2H), 5.17 (s, 2H); EI-MS: m/z 403 (M), 304, 225; HRMS: calculated for C23H17NO2S2, 403.0701, found 403.0708; HPLC Purity (retention time): 100% (4.95 min)
(Z)-2-(5-((4′-(benzyloxy)biphenyl-4-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (7Fc)
1H NMR (300 MHz, d6-DMSO): δ 7.90 (s, 1H), 7.88 (d, J=8.6 Hz, 2H), 7.76 (m, 4H), 7.34–7.48 (m, 5H), 7.15 (d, J=8.6 Hz, 2H), 5.18 (s, 2H), 4.75 (s, 2H); EI-MS: m/z 461 (M), 302, 225; HRMS: calculated for C25H19NO4S2, 461.0755, found 461.0762; HPLC Purity (retention time): 100% (4.09 min)
(Z)-5-((4′-(pyridin-3-ylmethoxy)biphenyl-4-yl)methylene)thiazolidine-2,4-dione (7Ga)
1H NMR (300 MHz, d6-DMSO): δ 8.72 (s, 1H), 8.58 (d, J=5.0 Hz, 1H), 7.97 (d, J=7.2 Hz, 1H), 7.82 (m, 3H), 7.67–7.74 (m, 4H), 7.48 (m, 1H), 7.15 (d, J=8.6 Hz, 2H), 5.24 (s, 2H); EI-MS: m/z 388 (M), 296, 289, 225; HPLC Purity (retention time): 98% (3.63 min)
(Z)-5-((4′-(pyridin-3-ylmethoxy)biphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (7Gb)
1H NMR (300 MHz, d6-DMSO): δ 8.73 (s, 1H), 8.59 (d, J=5.1 Hz, 1H), 7.97 (d, J=7.3 Hz, 1H), 7.81 (d, J=8.4 Hz, 2H), 7.67 (m, 5H), 7.50 (dd, J=7.3 Hz, J=5.1 Hz, 1H), 7.15 (d, J=8.8 Hz, 2H), 5.23 (s, 2H); EI-MS: m/z 404 (M), 262, 226; HPLC Purity (retention time): 96% (4.03 min)
(Z)-2-(4-oxo-5-((4′-(pyridin-3-ylmethoxy)biphenyl-4-yl)methylene)-2-thioxothiazolidin-3-yl)acetic acid (7Gc)
1H NMR (300 MHz, d6-DMSO): δ 8.73 (s, 1H), 8.58 (d, J=5.0 Hz, 1H), 7.98 (d, J=7.4 Hz, 1H), 7.91 (s, 1H), 7.89 (d, J=8.5 Hz, 2H), 7.79 (m, 4H), 7.52 (m, 1H), 7.18 (d, J=8.7 Hz, 2H), 5.24 (s, 2H), 4.76 (s, 2H); EI-MS: m/z 462 (M), 370, 302, 225; HPLC Purity (retention time): 99% (3.30 min)
(Z)-5-((4′-phenethoxybiphenyl-4-yl)methylene)thiazolidine-2,4-dione (7Ha)
1H NMR (300 MHz, d6-DMSO): δ 7.81 (m, 3H), 7.67–7.74 (m, 4H), 7.23–7.32 (m, 5H), 7.05 (d, J=8.7 Hz, 2H), 4.25 (t, J=6.8 Hz, 2H), 3.05 (t, J=6.8 Hz, 2H); EI-MS: m/z 401 (M), 302, 297, 226; HPLC Purity (retention time): 98% (4.44 min)
(Z)-5-((4′-phenethoxybiphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (7Hb)
1H NMR (300 MHz, d6-DMSO): δ 7.80 (d, J=8.5 Hz, 2H), 7.67 (m, 5H), 7.23–7.33 (m, 5H), 7.04 (d, J=8.9 Hz, 2H), 4.24 (t, J=6.9 Hz, 2H), 3.05 (t, J=6.9 Hz, 2H); EI-MS: m/z 417 (M), 313, 226; HPLC Purity (retention time): 96% (4.89 min)
(Z)-2-(4-oxo-5-((4′-phenethoxybiphenyl-4-yl)methylene)-2-thioxothiazolidin-3-yl)acetic acid (7Hc)
1H NMR (300 MHz, d6-DMSO): δ 7.89 (s, 1H), 7.87 (d, J=8.5 Hz, 2H), 7.76 (m, 4H), 7.25–7.34 (m, 5H), 7.05 (d, J=8.7 Hz, 2H), 4.75 (s, 2H), 4.25 (t, J=6.9 Hz, 2H), 3.05 (t, J=6.9 Hz, 2H); EI-MS: m/z 475 (M), 371, 226; HPLC Purity (retention time): 96% (4.05 min)
(Z)-5-((4′-(3-phenylpropoxy)biphenyl-4-yl)methylene)thiazolidine-2,4-dione (7Ia)
1H NMR (300 MHz, d6-DMSO): δ 7.81 (m, 3H), 7.67–7.75 (m, 4H), 7.18–7.30 (m, 5H), 7.04 (d, J=8.7 Hz, 2H), 4.02 (t, J=6.5 Hz, 2H), 2.75 (t, J=6.9 Hz, 2H), 2.01 (m, 2H); EI-MS: m/z 415 (M), 344, 297, 226; HPLC Purity (retention time): 98% (4.41 min)
(Z)-5-((4′-(3-phenylpropoxy)biphenyl-4-yl)methylene)-2-thioxothiazolidin-4-one (7Ib)
1H NMR (300 MHz, d6-DMSO): δ 7.81 (d, J=8.4 Hz, 2H), 7.67 (m, 5H), 7.18–7.29 (m, 5H), 7.04 (d, J=8.7 Hz, 2H), 4.01 (t, J=6.3 Hz, 2H), 2.75 (t, J=6.9 Hz, 2H), 2.03 (m, 2H); EI-MS: m/z 431 (M), 344, 313, 226; HPLC Purity (retention time): 99% (4.83 min)
(Z)-2-(4-oxo-5-((4′-(3-phenylpropoxy)biphenyl-4-yl)methylene)-2-thioxothiazolidin-3-yl)acetic acid (7Ic)
1H NMR (300 MHz, d6-DMSO): δ 7.89 (s, 1H), 7.87 (d, J=8.5 Hz, 2H), 7.76 (m, 4H), 7.18–7.30 (m, 5H), 7.05 (d, J=8.6 Hz, 2H), 4.75 (s, 2H), 4.03 (t, J=6.2 Hz, 2H), 2.75 (t, J=6.7 Hz, 2H), 2.04 (m, 2H); EI-MS: m/z 489 (M), 450, 332, 226; HPLC Purity (retention time): 97% (4.00 min)
Author contribution
Jing-kang SHEN and Jia LI designed the research; Zhang LIU, Qian CHAI, Yuan-yuan LI, Qiang Shen, Lan-ping MA, Jing-ya LI, Li-na ZHANG, and Li SHENG performed the research; Xin WANG contributed new analytical tools and reagents; Zhang LIU, Qian CHAI and Lan-ping MA analyzed data; Zhang LIU wrote the paper.
Acknowledgments
This work was supported by the 863 High-Tech Research and Development Program of China (Grant 2006AA02Z315 and 2008AA02Z105), the National S&T Major Projects “Key New Drug Creation and Manufacturing Program” of China (No 2009ZX09301-001, 2009ZX09302-001 and 2009ZX09501-010) and the Public Service Platform Foundation of Shanghai Ministry of Science and Technology (08DZ2291300 and 09DZ2291200).
References
- Hunter T. Signaling — 2000 and Beyond. Cell. 2000;100:113–27. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- Tonks NK, Neel BG. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol. 2001;13:182–95. doi: 10.1016/s0955-0674(00)00196-4. [DOI] [PubMed] [Google Scholar]
- Kappert K, Peters KG, Bohmer FD, Ostman A. Tyrosine phosphatases in vessel wall signaling. Cardio Vasc Res. 2005;65:587–98. doi: 10.1016/j.cardiores.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Stoker AW. Protein tyrosine phosphatases and signaling. J Endocrinol. 2005;185:19–33. doi: 10.1677/joe.1.06069. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Bio. 2006;7:833–46. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Kuo JC, Ku W, Lee YR, Lin FC, Chang YL, et al. The tumor suppressor dapk is reciprocally regulated by tyrosine kinase src and phosphatase LAR. Mol Cell. 2007;27:701–16. doi: 10.1016/j.molcel.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Zhang ZY. Protein tyrosine phosphatases: prospects for therapeutics. Curr Opin Chem Biol. 2001;5:416–23. doi: 10.1016/s1367-5931(00)00223-4. [DOI] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R, Bombrun A, Swinnen D. Selecting protein tyrosine phosphatases as drug targets. Drugs Discov Today. 2002;7:1013–9. doi: 10.1016/s1359-6446(02)02438-8. [DOI] [PubMed] [Google Scholar]
- Hendriks WJ, Elson A, Harroch S, Stoker AW. Protein tyrosine phosphatases: functional inferences from mouse models and human diseases. FEBS J. 2008;275:816–30. doi: 10.1111/j.1742-4658.2008.06249.x. [DOI] [PubMed] [Google Scholar]
- Julien SG. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary rumorigenesis and protects from lung metastasis. Nat Genet. 2007;39:338–46. doi: 10.1038/ng1963. [DOI] [PubMed] [Google Scholar]
- Johnson TO, Ermolieff J, Jirousek MR. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat Rev Drug Discov. 2002;1:696–709. doi: 10.1038/nrd895. [DOI] [PubMed] [Google Scholar]
- Liu G, Trevillyan JM. Protein tyrosine phosphatase 1B as a target for the treatment of impaired glucose tolerance and type II diabetes. Curr Opin Investig Drugs. 2002;3:1608–16. [PubMed] [Google Scholar]
- Tobin JF, Tam S. Recent advances in the development of small molecule inhibitors of PTP1B for the treatment of insulin resistance and type 2 diabetes. Curr Opin Drug Discov Devel. 2002;5:500–12. [PubMed] [Google Scholar]
- Zhang Z. Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development. Annu Rev Pharmacol Toxicol. 2002;42:209–34. doi: 10.1146/annurev.pharmtox.42.083001.144616. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhou B, Xie L. Modulation of protein kinase signaling by protein phosphatases and inhibitors. Pharmacol Therap. 2002;93:307–17. doi: 10.1016/s0163-7258(02)00199-7. [DOI] [PubMed] [Google Scholar]
- Kennedy BP, Ramachandran C. Protein tyrosine phosphatase-1B in diabetes. Biochem Pharmacol. 2000;60:877–83. doi: 10.1016/s0006-2952(00)00305-1. [DOI] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase 1B gene. Science. 1999;283:1544–8. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, et al. Increased energy expenditure decreased adiposity and tissue-specific insulin sensitivity in protein — tyrosine phosphatase 1B deficient mice. Mol Cell Biol. 2000;20:5479–89. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinker BA, Rondinone CM, Trevillyan JM, Gum RJ, Clampit JE, Waring JF. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc Natl Acad Sci USA. 2002;99:11357–62. doi: 10.1073/pnas.142298199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum RJ, Gaede LL, Koterski SL, Heindel M, Clampit JE, Zinker BA, et al. Reduction of protein tyrosine phosphatase 1b increases insulin-dependent signaling in ob/ob mice. Diabetes. 2003;52:21–8. doi: 10.2337/diabetes.52.1.21. [DOI] [PubMed] [Google Scholar]
- Lessard L, Stuible M, Tremblay ML. The two faces of PTP1B in cancer. Biochim Biophys Acta. 2010;1804:613–9. doi: 10.1016/j.bbapap.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhang ZY. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today. 2007;12:373–81. doi: 10.1016/j.drudis.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Lee S, Wan Q. Recent development of small molecular specific inhibitor of protein tyrosine phosphatase 1B. Med Res Rev. 2007;27:553–73. doi: 10.1002/med.20079. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li Y, Guo YW, Jiang HL, Shen X. A sesquiterpene quinone, dysidine, from the sponge Dysidea villosa, activates the insulin pathway through inhibition of PTPases. Acta Pharmacol Sin. 2009;30:333–45. doi: 10.1038/aps.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Yu HP, Zhou YY, Du JQ, Shen Q, Li JY, et al. Discovery of a novel competitive inhibitor of PTP1B by high-throughput screening. Acta Pharmacol Sin. 2008;29:278–84. doi: 10.1111/j.1745-7254.2008.00737.x. [DOI] [PubMed] [Google Scholar]
- Hartshorn MJ, Murray CW, Cleasby A, Frederickson M, Tickle IJ, Jhoti H. Fragment-based lead discovery using X-ray crystallography. J Med Chem. 2005;48:403–13. doi: 10.1021/jm0495778. [DOI] [PubMed] [Google Scholar]
- Wilson DP, Wan ZK, Xu WX, Kirincich SJ, Follows BC, Joseph-McCarthy D, et al. Structure-based optimization of protein tyrosine phosphatase 1B inhibitors: from the active site to the second phosphotyrosine binding site. J Med Chem. 2007;50:4681–98. doi: 10.1021/jm0702478. [DOI] [PubMed] [Google Scholar]
- Hussain M, Ahmed V, Hill B, Ahmed Z, Taylor SD. A re-examination of the difluoromethylenesulfonic acid group as a phosphotyrosine mimic for PTP1B inhibition. Bioorg Med Chem. 2008;16:6764–77. doi: 10.1016/j.bmc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- Reddy KA, Lohray BB, Bhushan V, Bajji AC, Reddy KV, Reddy PR, et al. Novel antidiabetic and hypolipidemic agents. 3. Benzofuran-containing thiazolidinediones. J Med Chem. 1999;42:1927–40. doi: 10.1021/jm980549x. [DOI] [PubMed] [Google Scholar]
- Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–50. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- Costantino L, Rastelli G, Vianello P, Cignarella G, Barlocco D. Diabetes complications and their potential prevention: aldose reductase inhibition and other approaches. Med Res Rev. 1999;19:3–23. doi: 10.1002/(sici)1098-1128(199901)19:1<3::aid-med2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Malamas MS, Sredy J, Gunawan I, Mihan B, Sawicki DR, Seestaller L, et al. New azolidinediones as inhibitors of protein tyrosine phosphatase 1B with antihyperglycemic properties. J Med Chem. 2000;43:995–1010. doi: 10.1021/jm990476x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Huang Y, Zhang W, Ma LP, Li JY, Wang X, et al. Soluble polymer-supported synthesis of 5-arylidene thiazolidinones and pyrimidinones using a novel traceless linker strategy. J Comb Chem. 2008;10:632–6. doi: 10.1021/cc800054e. [DOI] [PubMed] [Google Scholar]
- Zhang W, Hong D, Zhou Y, Zhang Y, Shen Q, Li JY, et al. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim Biophys Acta. 2006;1760:1505–12. doi: 10.1016/j.bbagen.2006.05.009. [DOI] [PubMed] [Google Scholar]