Abstract
Allogeneic stem cell transplantation is the standard approach to Philadelphia chromosome positive acute lymphoblastic leukemia. We hypothesized that imatinib plus sequential chemotherapy will result in significant leukemia cell cytoreduction in patients with Philadelphia chromosome positive acute lymphoblastic leukemia, allowing collection of normal hematopoietic stem cells uncontaminated by residual BCR/ABL1+ lymphoblasts and thus reduce the likelihood of relapse after autologous stem cell transplantation for patients under 60 years of age without sibling donors. We enrolled 58 patients; 19 underwent autologous and 15 underwent allogeneic stem cell transplantation on study. Imatinib plus sequential chemotherapy resulted in reverse-transcriptase polymerase chain reaction-negative stem cells in 9 patients and remained minimally positive in 4 (6 were not evaluable). Overall survival (median 6.0 years vs. not reached) and disease-free survival (median 3.5 vs. 4.1 years) were similar between those who underwent autologous and those who underwent allogeneic stem cell transplantation. We conclude that autologous stem cell transplantation represents a safe and effective alternative for allogeneic stem cell transplantation in Philadelphia chromosome positive acute lymphoblastic leukemia patients without sibling donors (clinicaltrials.gov identifier:00039377).
Introduction
Imatinib mesylate has significantly improved the response rate, and disease-free and overall survival (OS) for patients with Philadelphia chromosome-positive (Ph+; BCR/ABL1+) acute lymphoblastic leukemia (ALL). Three studies have demonstrated a clear survival benefit for post-induction allogeneic (allo)-stem cell transplantation (SCT) over chemotherapy alone with imatinib.1 The optimal treatment for patients who are not eligible for HLA-matched allo-SCT, however, remains controversial. In the Cancer and Leukemia Group B (CALGB) study 10001, patients under 60 years of age with matched sibling donors proceeded to an allo-SCT and those without matched sibling donors were offered autologous (auto)-SCT to test the hypothesis that imatinib and sequential chemotherapy will result in significant leukemia cell cytoreduction allowing collection of large numbers of normal hematopoietic stem cells uncontaminated by residual BCR/ABL1+ lymphoblasts and thus reduce the likelihood of relapse after auto-SCT using an intensive preparative regimen.
Methods
Patients
Patients were eligible if they were 15 years of age or over and under 60 years of age, had an unequivocal diagnosis of t(9;22) or BCR/ABL1+ ALL, had achieved either partial or complete remission (CR) following one course of induction chemotherapy with a 4- or 5-drug regimen, and had received imatinib for no more than six weeks before study enrollment (Online Supplementary Tables S1 and S2). All patients provided written informed consent. The study received Institutional Review Board approval from each participating institution. Between April 15th 2002 and April 30th 2010, 58 patients were enrolled; one was ineligible. Twenty-two patients were taken off study for alternative donor transplants or other reasons (see CONSORT diagram in the Online Supplementary Appendix). One patient was treated and followed without a transplant. The median OS of these 23 patients was 1.9 years. Disease-free survival (DFS) and OS of the whole cohort are shown in the Online Supplementary Figure S1A and B. The following analysis compares the outcomes of the 34 patients who underwent allo- (n=15) or auto- (n=19) SCT on this study.
Treatment protocol
Details of the treatment protocol are available in the Online Supplementary Appendix.
Karyotype
The diagnosis of Ph+ ALL was based on the analysis of 20 or more metaphases in bone marrow specimens and confirmed by central karyotype review.2
Real-time polymerase chain reaction and mutation analysis
Real-time polymerase chain reaction (RT-PCR) was performed in a central CALGB laboratory as previously described.3 Sequencing for ABL1 kinase domain mutation analyses was performed as previously described4 with the following forward primer being used to amplify the p190 BCR/ABL1 transcript: 5′-ACCATCGTGGGCGTCCGCAAGA-3′.
Response criteria
Previously established criteria were used for definitions of hematologic CR, partial response (PR), OS and disease-free survival (DFS).5 Complete response was defined as recovery of morphologically normal bone marrow and blood counts (i.e. neutrophils ≥1.5×109/L and platelets >100×109/L), and no circulating leukemic blasts or evidence of extramedullary leukemia, and persisting for at least one month. PR required all of the CR criteria except that the marrow may still contain 5–25% blasts. Disease-free survival was measured from the date of CR until the date of relapse or death; patients alive and in CR were censored at last follow up. Overall survival was measured from the date of study entry until the date of death, and patients alive at last follow up were censored. Cumulative incidence of relapse was calculated from date of documented CR to ALL relapse (bone marrow or extramedullary) or death. Patients without relapse were uninformatively censored, whereas those who experienced treatment-related mortality were counted as a competing cause of failure.6,7
Major molecular response (MMR) and complete molecular response (CMR) were defined as previously described.8
Statistical methods
The study was designed to test the hypothesis that the median DFS for Ph+ ALL would exceed one year9 and to compare outcomes following auto-SCT and allo-SCT. The planned sample size was 60 patients. Given the total sample size of 34, the power to reject the hypothesis was 0.73. The type II error was 1-0.73=0.27. The Kaplan-Meier estimator and the log rank test were used for survival analyses. The inferential results are two-sided and have not been adjusted to account for multiple testing. All analyses were performed by the Alliance for Clinical Trials in Oncology Statistics and Data Center.
Results
Characteristics of the whole cohort by transplant type are described in Table 1 and adverse events are described in Online Supplementary Table S3. The cohorts differed by their Eastern Cooperative Oncology Group performance status (P=0.0075) at study entry and time to transplant (P<0.0001) (Table 1) but not in their induction treatments (Online Supplementary Table S1). Stem cell mobilization was successful in all 19 patients; the median autologous CD34+ cell yield by leukapheresis was 70.2 (range 4.3–309.8) × 106/kg. Peripheral blood stem cells were assayed from 13 patients by RT-PCR with a sensitivity of 1:105–106; 9 (64%) had no detectable BCR-ABL1 (CMR); 4 had minimally detectable BCR-ABL1 (MMR); 6 were not evaluable due to poor stem cell collection or because data concerning the initial transcript was not available. The RT-PCR status of the stem cell products had no effect on OS or DFS after auto-SCT (Figure 1A and B) although this may be related to the small number of cases.
Table 1.
Patients’ characteristics and outcome by transplant type
Figure 1.
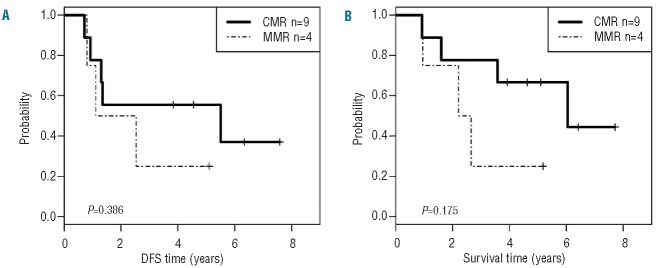
Disease-free (A) and overall survival (B) stratified by BCR-ABL status of the peripheral blood stem cell collection measured by Q-RT-PCR. CMR: complete molecular response; DFS: disease-free survival; MMR: major molecular response.
Treatment-related mortality (TRM; defined here as death before Day 100) associated with auto-SCT occurred in one (5%) of 19 patients, while TRM associated with allo-SCT occurred in 3 (20%) of 15 patients. There was no statistical difference in TRM between the two SCT modalities (P=0.3); again, this may be due to the small numbers in this study. The TRM following allo-SCT is within the range of TRM reported by other groups in this patient population undergoing a myeloablative preparatory regimen.10–12
A total of 8 patients (3 post auto-SCT and 5 post allo-SCT) converted from MMR to CMR after SCT. The effect of minimal residual disease (MRD) on outcome following auto-SCT was studied. DFS and OS of patients who achieved at least an MMR (n=8) at Day +120 (only one achieved a CMR) was longer than those for patients who did not achieve an MMR at that time point (P=0.09 and P=0.026, respectively) (Figure 2A and B). As far as allo-SCT is concerned, 7 of 10 patients who survived 120 days had CMR; 2 additional patients achieved MMR and one patient had residual MRD over 0.001. However, the sample size was too small to analyze the effect of MRD on outcome. Overall, these data suggest that patients who have MRD at levels lower than or equal to MMR following auto-SCT have prolonged survival. This may result from additional exposure to imatinib maintenance following auto-SCT.
Figure 2.
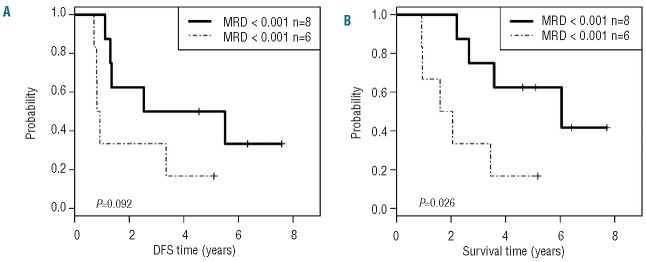
Disease-free (A) and overall (B) survival, stratified by minimal residual disease (MRD) at Day +120 following autologous stem cell transplant (SCT); MRD ≤0.001 (major molecular response) versus >0.001 (lack of major molecular response).
Seven (47%) of the allo-SCT patients remain alive in CR and 8 (42%) of the auto-SCT patients remain alive in CR (see CONSORT Diagram in the Online Supplementary Appendix). The median survival of the living patients was 4.8 years. Ten of the transplanted patients have relapsed (8 following auto-SCT and 2 following allo-SCT). Relapses occurred at a median of 5.9 months following auto-SCT.13 There was no statistical difference in relapse rates between the two SCT modalities (P=0.1285). An ABL1 kinase mutation was detected in 7 (70%) of the 10 patients on whom samples were available at relapse (Online Supplementary Table S4).
Discussion
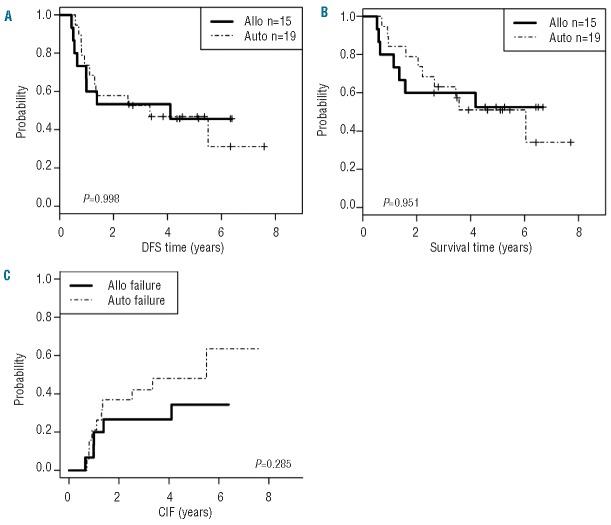
Our results with Ph+ ALL demonstrate similar outcomes for auto-SCT and allo-SCT (Figure 3A–C). The difference between our results and those of Goldstone et al.14 likely stem from the ability of imatinib to purge the marrow prior to stem cell collection, the intensity of the preparative regimen, and the ability to apply maintenance TKI therapy following auto-SCT. Our results are supported by several other publications15–20 demonstrating success of auto-SCT in Ph+ ALL. The availability of more potent ABL1 kinase inhibitors offers the potential for even better outcomes. Interestingly, the LAL 1205 trial evaluated dasatinib combined with steroids for induction.21 That study demonstrated an OS of 69% and DFS of 51% at 20 months, although 32 of the 53 (60%) patients underwent allo-SCT, auto-SCT or additional chemotherapy in first CR, suggesting that dasatinib and steroids alone may not be sufficient to achieve long-term DFS. This is further supported by the results from the recent publication of the trial combining dasatinib and hyper-CVAD; there was no plateau in the survival curve possibly because only a few patients underwent SCT.22 The use of dasatinib for remission induction followed by allo-SCT or auto-SCT in Ph+ ALL is currently being evaluated in the North American Leukemia Intergroup study CALGB 10701 (Alliance). The rationale to replace imatinib by dasatinib is based on its more potent activity, resistance to most mutations, and activity against Src kinases believed to play a role in Ph+ ALL but not in chronic myeloid leukemia.23 In summary, we demonstrate that auto-SCT represents a safe and effective alternative for allo-SCT in Ph+ ALL patients without matched donors, although SCT from alternative donors was not tested in this study.
Figure 3.
Disease-free (A), overall survival (B) and cumulative incidence of failure (CIF) (C) stratified by transplant type; autologous (auto) stem cell transplant (SCT) versus allogeneic (allo) SCT.
Acknowledgments
The trial was partially supported by the following grants: CA059518, CA33601, CA41287; CA31946, CA 77658, CA101140, Leukemia Clinical Research Foundation, CA138561.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Lee HJ, Thompson JE, Wang ES, Wetzler M. Philadelphia chromosome-positive acute lymphoblastic leukemia: current treatment and future perspectives. Cancer. 2011;117(8):1583–94 [DOI] [PubMed] [Google Scholar]
- 2.Wetzler M, Dodge RK, Mrozek K, Stewart CC, Carroll AJ, Tantravahi R, et al. Additional cytogenetic abnormalities in adults with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a study of the Cancer and Leukaemia Group B. Br J Haematol. 2004;124(3):275–88 [DOI] [PubMed] [Google Scholar]
- 3.Stock W, Yu D, Karrison T, Sher D, Stone RM, Larson RA, et al. Quantitative realtime RT-PCR monitoring of BCR-ABL in chronic myelogenous leukemia shows lack of agreement in blood and bone marrow samples. Int J Oncol. 2006;28(5):1099–103 [PubMed] [Google Scholar]
- 4.Soverini S, Martinelli G, Amabile M, Poerio A, Bianchini M, Rosti G, et al. Denaturing-HPLC-based assay for detection of ABL mutations in chronic myeloid leukemia patients resistant to Imatinib. Clin Chem. 2004;50(7):1205–13 [DOI] [PubMed] [Google Scholar]
- 5.Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995;85(8):2025–37 [PubMed] [Google Scholar]
- 6.Kalbfleisch JD, Pentice RL. The statistical analysis of failure time data. 2nd Edition ed: Wiley-interscience, 2002 [Google Scholar]
- 7.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–54 [Google Scholar]
- 8.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wetzler M, Dodge RK, Mrozek K, Carroll AJ, Tantravahi R, Block AW, et al. Prospective karyotype analysis in adult acute lymphoblastic leukemia: the cancer and leukemia Group B experience. Blood. 1999;93(11):3983–93 [PubMed] [Google Scholar]
- 10.Mizuta S, Matsuo K, Yagasaki F, Yujiri T, Hatta Y, Kimura Y, et al. Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia. Leukemia. 2011;25(1):41–7 [DOI] [PubMed] [Google Scholar]
- 11.Blume KG, Forman SJ, O’Donnell MR, Doroshow JH, Krance RA, Nademanee AP, et al. Total body irradiation and high-dose etoposide: a new preparatory regimen for bone marrow transplantation in patients with advanced hematologic malignancies. Blood. 1987;69(4):1015–20 [PubMed] [Google Scholar]
- 12.Marks DI, Forman SJ, Blume KG, Perez WS, Weisdorf DJ, Keating A, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12(4):438–53 [DOI] [PubMed] [Google Scholar]
- 13.Koval G, Wetzler M, Watson D, Owzar K, Bloomfield CD, Malnassy G, et al. Abl Kinase Domain Mutations Leading to Relapse of Ph+ Acute Lymphoblastic Leukemia (ALL) Occur Commonly and Can Be Detected At Initial Diagnosis: Molecular Results From CALGB 10001. ASH Annual Meeting Abstracts. 2011;118(21):2541–. [Google Scholar]
- 14.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia (ALL) the greatest benefit is achieved from a matched sibling allogeneic transplant in first complete remission (CR) and an autologous transplant is less effective than conventional consolidation/maintenance chemotherapy in All patients: final results of the international ALL trial (MRC UKALL XII/ ECOG E2993). Blood. 2008;111(4):1827–33 [DOI] [PubMed] [Google Scholar]
- 15.Shin HJ, Chung JS, Cho GJ. Imatinib interim therapy between chemotherapeutic cycles and in vivo purging prior to autologous stem cell transplantation, followed by maintenance therapy is a feasible treatment strategy in Philadelphia chromosome-positive acute lymphoblastic leukemia. Bone Marrow Transplant. 2005;36(10):917–8 [DOI] [PubMed] [Google Scholar]
- 16.Ribera JM, Oriol A, Gonzalez M, Vidriales B, Brunet S, Esteve J, et al. Concurrent intensive chemotherapy and imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Final results of the CSTIBES02 trial. Haematologica. 2010;95(1):87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanguy-Schmidt A, de Labarthe A, Rousselot P, Huguet F, Delabesse E, Witz F, et al. Long-Term Results of the Imatinib GRAAPH-2003 Study in Newly-Diagnosed Patients with De Novo Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. ASH Annual Meeting Abstracts. 2009;114(22):3080–. [Google Scholar]
- 18.Linker C, Damon L, Martin T, Blume K, Forman S, Snyder D, et al. Autologous hematopoietic cell transplantation for high-risk ALL. Bone Marrow Transplant. 2011;46(3):460–1 [DOI] [PubMed] [Google Scholar]
- 19.Bassan R, Rossi G, Pogliani EM, Di Bona E, Angelucci E, Cavattoni I, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28(22):3644–52 [DOI] [PubMed] [Google Scholar]
- 20.Giebel S, Labopin M, Gorin NC, Caillot D, Leguay T, Schaap N, et al. Autologous HSCT for Ph-Positive Adult Acute Lymphoblastic Leukemia: A Curative Option in the Era of Tyrosine Kinase Inhibitors? an Analysis From the Acute Leukemia Working Party of the EBMT. ASH Annual Meeting Abstracts. 2012;120(21):233–. [Google Scholar]
- 21.Foa R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521–8 [DOI] [PubMed] [Google Scholar]
- 22.Ravandi F, O’Brien S, Thomas D, Faderl S, Jones D, Garris R, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116(12):2070–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, Liu Y, Pelletier S, Buchdunger E, Warmuth M, Fabbro D, et al. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat Genet. 2004;36(5):453–61 [DOI] [PubMed] [Google Scholar]