Diabetic angiopathies, such as cardiovascular diseases, cerebrovascular diseases and nephropathy, are major causes of death in diabetic patients. Diabetic retinopathy is not a direct cause of death in these patients, but is a major threat as a cause of blindness in the working‐age diabetic population. Several studies have suggested that rigid glycemic control prevents the progression and deterioration of a microangiopathy such as retinopathy. However, the Diabetes Control and Complications Trial and follow‐up the Epidemiology of Diabetes Interventions and Complications studies showed that persistent tight glycemic control in diabetic patients does not provide short‐term prevention or amelioration of diabetic retinopathy, but inhibits and improves this condition beyond the period of good glycemic control1. This phenomenon is referred to as ‘metabolic memory’ or a ‘legacy effect’, and has also been observed for macroangiopathies, including cardiovascular diseases2.
Maintenance of good glycemic control over a long period is difficult for some patients with diabetes, especially in the elderly population. Thus, development of drugs based on the pathogenesis of diabetic retinopathy is needed, but no such drugs are available for use in early stage diabetic retinopathy. The recent study of Gerhardinger et al.3 is very encouraging in this respect, because mechanisms that might allow management of diabetic retinopathy were identified. The aim of that study was to identify candidate drug targets by investigating the molecular effects of drugs that prevent loss of retinal capillaries in diabetic rats. The gene expression profile of retinal vessels isolated from rats 6 months after onset of streptozotocin‐induced diabetes was compared with that of control rats. In addition to genes involved in oxidative stress, inflammation, vascular remodeling and apoptosis, the differential expression of 20 genes of the transforming growth factor (TGF)‐β pathway was observed in the retinal vessels of diabetic rats. Furthermore, the complete loop of TGF‐β signaling, including Smad 2 phosphorylation, was enhanced in the retinal vessels.
Sorbinil, an inhibitor of aldose reductase (a key enzyme of the polyol pathway), normalized the expression of 71% of the genes related to oxidative stress and 62% of those related to inflammation, whereas aspirin had a minimal or no effect on expression of these genes. However, sorbinil and aspirin had similar effects in reducing upregulation of genes of the TGF‐β pathway by 55 and 40%, and those involved in apoptosis by 74 and 42%, respectively. Based on these results, it was concluded that oxidative and inflammatory stress is the distinct signature of the polyol pathway in retinal vessels and that TGF‐β and apoptosis are targets for prevention of capillary loss in diabetic retinopathy. Determination of similar characteristics in human diabetes will show whether there is a rationale for adjunct therapy with aldose reductase inhibitors.
Despite extensive studies, diabetic retinopathy remains difficult to prevent and treat. Retinal photocoagulation was introduced for this purpose over half a century ago and is still used for the management of diabetic retinopathy. However, this treatment is not always effective in restoring visual loss in advanced cases. Development of a suitable treatment in the early or mild stage of diabetic retinopathy is desirable for the prevention of progression to the severe stage. Thus, the results of Gerhardinger et al.3 suggest the possibility of drug development based on the pathogenesis of diabetic retinopathy.
Diabetic complications, including diabetic retinopathy, have been proposed to be as a result of metabolic factors, such as oxidative stress, changes in the PKC, glycation and polyol pathways, and inflammation. Oxidative stress is particularly important in the pathogenesis of diabetic retinopathy4,5. In the study by Gerhardinger et al.3, sorbinil inhibited the elevation of expression of genes associated with oxidative stress and inflammation, and those involved in the TGF‐β pathway and apoptosis in retinal vessels of diabetic rats. Sorbinil inhibits aldose reductase, which is a key enzyme of the polyol pathway. This shows that suppression of the polyol pathway as the gate to hyperglycemia‐induced metabolic changes is very important in the prevention of diabetic retinopathy at an early or mild stage. It also suggests that the inhibition of a common metabolic pathway can occur downstream of the hyperglycemia‐induced changes (Figure 1).
Figure 1.

Hyperglycemia alters the expression of multiple genes of the transforming growth factor‐β (TGF‐β) pathway through the polyol pathway in retinal vessels. Upregulated genes are shown in red and downregulated genes are shown in blue. The expected effect of overexpression of genes on Smad signaling is shown by arrowed lines (stimulation) or blunt lines (inhibition). The expected effect of downregulation of Hoxc8 is release of inhibition, and thus activation of signaling (dotted blunt line). BMP, bone morphogenetic proteins. The figure is a modification from the original in Gerhardinger et al.3
In our 3‐year multicenter, randomized, controlled study (the Aldose Reductase Inhibitor‐Diabetes Complications Trial [ADCT]), epalrestat, an aldose reductase inhibitor, significantly reduced deterioration of nerve function in patients with subjective symptoms6. The effect of this drug on diabetic retinopathy was observed as a secondary end‐point. In subjects with no or simple retinopathy at baseline, an improvement at year 3 was seen in one of 114 (0.88%) and seven of 99 (7.07%) subjects in the control and epalrestat groups, respectively. In contrast, 19 (16.67%) and 10 (10.10%) subjects showed deterioration in retinopathy in the control and epalrestat groups, respectively. The difference in improvement and deterioration rates between the groups was statistically significant (P = 0.026) (Figure 2). In our previous open trial of 3 years, a similar result for treatment with epalrestat was observed. In a small, short‐term study (6 months), it has also been reported that sorbinil prevents deterioration of diabetic retinopathy. Therefore, aldose reductase inhibitors might be effective for preventing onset and delaying progression of simple retinopathy in patients with diabetes.
Figure 2.
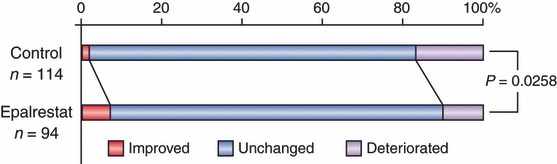
Long‐term clinical efficacy of epalrestat, an aldose reductase inhibitor, on diabetic retinopathy after 3 years. Diabetic retinopathy was evaluated by fundoscopy in subjects with no or simple retinopathy at baseline. The figure is a modification from the original in Hotta et al.6
TGF‐β is a pleiotropic growth factor that regulates cell growth and differentiation, apoptosis, cell motility, extracellular matrix production, angiogenesis and cellular immune responses. Although the TGF‐β signaling pathway plays a pivotal role in diverse cellular process, TGF‐β switches its role from a tumor suppressor to a tumor promotor in advanced cancers. Thus, TGF‐β signaling is considered to be a useful therapeutic target for cancer7.
In the study by Gerhardinger et al.3 attenuation of increased activity of the TGF‐β pathway and apoptosis caused by hyperglycemia appears to be required to prevent diabetic retinopathy, rather than attenuation of oxidative stress and inflammation. In our previous in vitro study using bovine pericytes, SNK‐860, an aldose reductase inhibitor, prevented deterioration through high glucose‐induced apoptosis8. This suggests that the polyol pathway might be an interesting target for treatment of diabetic retinopathy. Viewed against the lack of appreciable advances in the treatment of diabetic retinopathy, the findings of Gerhardinger et al.3 are clearly of interest and importance. The suppression of hyperglycemia induced‐hyperactivity of the polyol pathway in the upstream of other metabolic factors by aldose reductase inhibitors might play an important role in the prevention of diabetic retinopathy.
For the next step of drug development, it will be important for the hypothesis of the importance of the TGF‐β pathway in diabetic retinopathy to be examined by other groups using different approaches. The progression of vascular pathogenesis caused by excessive TGF‐β signaling has recently been shown to be reduced by angiotensin II blockers9, and the AKR1B1 (aldo‐keto reductase family 1, member 1) gene, which codes for an aldose reductase that is activated by high glucose, is a TGF‐β1 responsive gene that has been linked to chronic diabetic complications such as nephropathy10. These results add to the findings of a hyperactive TGF‐β pathway in diabetic retinopathy3. TGF‐β inhibitors have entered clinical trials and have shown promise in a variety of different tumor models in preclinical studies. Because orally active inhibitors of TGF‐β signaling are in development11, one of the steps proposed by Gerhardinger et al.3 is to define the effects of selective normalization of TGF‐β signaling in retinal vessels, with the goals of ascertaining whether the TGF‐β pathway is overactive in human diabetic retinopathy and if the polyol pathway plays an important role in the development of this disease.
Acknowledgement
There is no conflict of interests for the author.
References
- 1.The Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Effects of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002; 287: 2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular diseases in patients with type 1 diabetes. N Engl J Med 2005; 25: 2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerhardinger C, Dagher Z, Sebastiani P, et al. The transforming growth factor‐β pathway is a common target of drugs that prevent experimental diabetic retinopathy. Diabetes 2009; 58: 1650–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzi M. Mechanisms and strategies for prevention in diabetic retinopathy. Curr Diab Rep 2006; 6: 102–107 [DOI] [PubMed] [Google Scholar]
- 5.Kowluru RA, Chan P‐S. Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007; Article ID 43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotta N, Akanuma Y, Kawamori R, et al. Long‐term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy. Diabetes Care 2006; 29: 1538–1544 [DOI] [PubMed] [Google Scholar]
- 7.Nagaraj NS, Datta PK. Targeting the transforming growth factor‐beta signaling pathway in human cancer. Expert Opin Investig Drugs 2010; 19: 77–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naruse K, Nakamura J, Hamada Y, et al. Aldose reductase inhibition prevents glucose‐induced apoptosis in cultured bovine retinal microvascular pericytes. Exp Eye Res 2000; 71: 309–315 [DOI] [PubMed] [Google Scholar]
- 9.Brooke BS, Habashi JP, Judge DP, et al. Angiotensin II blockade and aortic‐root dilation in Marfan’s syndrome. N Engl J Med 2008; 358: 2787–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez AP, Sharma K. Transcription factors in the pathogenesis of diabetic nephropathy. Expert Rev Mol Med 2009; 11: e13 [DOI] [PubMed] [Google Scholar]
- 11.Fu K, Corbey MJ, Sun L, et al. SM 16 an orally active TGF‐β type 1 receptor inhibitor prevents myofibroblast induction and vascular fibrosis in the rat carotid injury model. Arterioscler Thromb Vasc Biol 2008; 28: 665–671 [DOI] [PubMed] [Google Scholar]


