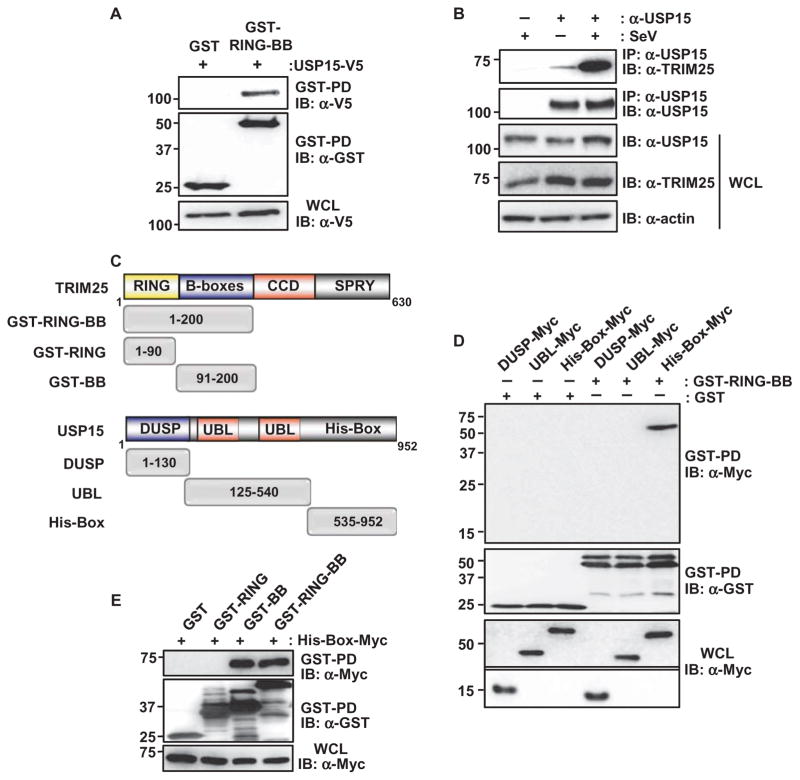
Fig. 1. USP15 interacts with TRIM25.
(A) HEK 293T cells were transfected with plasmids encoding GST or GST-TRIM25-RING-BB together with plasmid encoding USP15-V5. Forty-eight hours later, cells were lysed and whole-cell lysates (WCLs) were subjected to GST pull-down (GST-PD) and Western blotting (IB) analysis with anti-V5 antibody (α-V5) and anti-GST antibody (α-GST). Molecular mass markers (kD) are indicated to the left of the blots. (B) HEK 293T cells were left uninfected or were infected with SeV (50 HA U/ml). WCLs were prepared and subjected to immunoprecipitation (IP) with anti-USP15 antibody (α-USP15), and samples were analyzed by Western blotting with anti-TRIM25 antibody (α-TRIM25) and α-USP15 antibody. (C) Domain structures of TRIM25 and USP15, as well as a schematic representation of the GST-fused or Myc-tagged truncation mutants of TRIM25 and USP15, respectively. Numbers indicate amino acid residues. (D) HEK 293T cells were transfected with plasmids encoding the Myc-tagged DUSP, UBL, or His-Box domains of USP15 together with plasmids encoding GST or GST-TRIM25-RING-BB. Forty-eight hours later, WCLs were prepared and subjected to GST pull-down and Western blotting analysis with anti-Myc antibody (α-Myc) and α-GST antibody. WCLs were also analyzed by Western blotting with α-Myc. The upper part of this panel was exposed for a longer time than was used for the lower part. (E) HEK 293T cells were transfected with plasmids encoding GST or the indicated GST fusion constructs together with plasmid encoding Myc-tagged USP15 His-Box. Forty-eight hours later, WCLs were prepared and subjected to GST pull-down analysis, which was followed by Western blotting analysis with α-Myc and α-GST antibodies. Data in all panels are representative of three independent experiments.