Abstract
Vocal individuality and stability has been used to conduct population surveys, monitor population dynamics, and detect dispersal patterns in avian studies. To our knowledge, it has never been used in these kinds of studies among primates. The cao vit gibbon is a critically endangered species with only one small population living in a karst forest along China-Vietnam border. Due to the difficult karst terrain, an international border, long life history, and similarity in male morphology, detailed monitoring of population dynamics and dispersal patterns are not possible using traditional observation methods. In this paper, we test individuality and stability in male songs of cao vit gibbons. We then discuss the possibility of using vocal individuality for population surveys and monitoring population dynamics and dispersal patterns. Significant individuality of vocalization was detected in all 9 males, and the correct rate of individual identification yielded by discriminant function analysis using a subset of variables was satisfactory (>90%). Vocal stability over 2–6 years was also documented in 4 males. Several characters of cao vit gibbons allowed long-term population monitoring using vocal recordings in both China and Vietnam: 1) regular loud calls, 2) strong individuality and stability in male songs, 3) stable territories, and 4) long male tenure. During the course of this research, we also observed one male replacement (confirmed by vocal analysis). This time- and labor-saving method might be the most effective way to detect dispersal patterns in this transboundary population.
Introduction
Vocal communication is very common in birds, primates, and cetaceans that are living in dense forest or under water where olfactory and visual signals are limited or not feasible. For signal receivers, identification of the caller serves both to discriminate members from intruders and to maintain affiliative relationships with other individuals [1]. Signalers are expected to actively broadcast their identity with distinctive cues provided there is a benefit to being accurately identified [2]. Accordingly, vocal individuality has been demonstrated widely among different animal taxa [2]–[6], and it has also been used to evaluate territorial boundaries, map home ranges, conduct population surveys, monitor population dynamics, and detect dispersal patterns [7]–[11]. However, long-term stability of vocal individuality is a prerequisite for its utilization in these techniques [9].
Gibbons are small, arboreal, territorial apes, inhabiting the broad-leaved evergreen forests of Southeast Asia. They typically live in small groups comprised of one adult pair and 2–3 offspring [12]. All gibbons produce species-specific and sex-specific songs lasting 10–30 min in the early morning, and paired mates often combine their respective vocalizations into well-coordinated duets (with the exceptions of Hylobates klossii and H. moloch) [13], [14]. Several functional explanations of gibbon songs in which selection may have favored acoustic individuality and individual recognition have been proposed; these include regulating spacing among groups, defense of resources, mate attraction, and strengthening or advertising the pair bond [1], [3], [13], [15]–[17]. Although acoustic individuality has already been reported in several gibbon species: Hylobates agilis [1], [3], H. klossii [18], H. moloch [19], [20] and N. concolor [21], the vocal stability of gibbon songs has seldom been assessed (but see Fan et al. [22]).
In this study, we tested the vocal individuality and stability of male songs from cao vit gibbons (Nomascus nasutus) over the course of 2–6 years. Here we discuss the possibility of using vocal individuality to monitor the population dynamics of the species. Cao vit gibbons, also known as eastern black crested gibbons, are a Critically Endangered species [23] and listed as one of the ‘World's 25 Most Endangered Primates’ [24]. There is only one population remaining, with about 110 individuals surviving in a karst forest patch crossing the China-Vietnam border [25]. Accurate population estimates and monitoring are essential for the conservation of the species; however, the karst forest in which they live hampers close following and habituation. Researchers are rarely able to observe them from distances of less than 50 m [22], [26]. Adult females could be easily distinguish based on different shapes of their obvious white face ring and large black crown patch, but the adult males are almost entirely black without obvious distinguishing marks [27], making males difficult to identify easily in the forest. Capturing and handling these endangered gibbons safely would be practically impossible and would not likely be allowed by authorities. The international border splitting their habitat makes it difficult to monitor the whole population, as it is not possible to follow individuals that disperse across the border. Therefore, a time- and labour-saving method that could be used easily in both countries is needed to conserve the species. Using vocal individuality would be the most effective way to identify individuals in this situation [9].
Methods
Ethics Statement
No animals were handled during this study. Research permissions were issued by Mr. Tan Wu-Jing, the director of Bangliang Nature Reserve, and Mr. Nong Van Tao, the director of Cao Vit Gibbon Conservation Area. All work was done in accordance with guidelines of the national anthorities of both China and Vietnam.
Study Sites and Subject Animals
We carried out this study at two sites that form a contiguous zone across the border of China and Vietnam [28]: Bangliang Nature Reserve (22°55′N, 106°29′-30′E), Jingxi County, Guangxi, China and The Cao Vit Gibbon Conservation Area (22°54′-55′N, 106°31′-32′E), Trung Khanh, Cao Bang, Vietnam. The area is dominated by tropical monsoon forest at the center of the karst limestone block, and surrounded by degraded secondary forest and scrub [28].
Since 2008, only three gibbon groups have lived in Bangliang Nature Reserve [29], all of which were chosen for this study. G1 consisted of an adult male (G1 male) and a juvenile male in 2007. The juvenile male started to sing solo bouts in 2011 and dispersed in August 2013. We named it G1 subadult male in this study. A new male (G1 new male) replaced the G1 male between May and September 2012. G2 consisted of only one adult male and no juvenile or subadult males. G4 also consisted of an adult male (G4 male) and a juvenile male in 2007. The juvenile male developed into a subadult (G4 subadult male) and produced solo bouts in 2011, then dispersed in 2012. In total, we recorded solo or duet bouts from six different males in these three groups all of whom have been identified by morphological and behavioral characters (G1 male, G1 new male, G1 subadult male, G2 male, G4 male and G4 subadult male) (Table 1). Each of these three study groups has been intensively studied since 2007 and their home ranges are well known [26], [29]–[31].
Table 1. Sample size for 9 male cao vit gibbons in this study.
| Individuals | Number of male phrases/(Number of song bouts) | Total | ||||
| 2007 | 2008 | 2009 | 2011 | 2012 | ||
| G1 male | 8/(1) | 42/(7) | 4/(1) | 11/(5) | 17/(3) | 82/(17) |
| G1 new male | 66/(16) | 66/(16) | ||||
| G1 subadult male | 15/(2) | 85/(14) | 100/(16) | |||
| G2 male | 10/(2) | 22/(3) | 38/(5) | 56/(14) | 8/(3) | 134/(27) |
| G4 male | 6/(1) | 54/(6) | 30/(5) | 90/(12) | ||
| G4 subadult male | 22/(3) | 22/(3) | ||||
| V1 | 30/(6) | 30/(6) | ||||
| V2 | 11/(3) | 11/(3) | ||||
| V3 | 46/(10) | 46/(10) | ||||
| Total | 18/(3) | 64/(10) | 48/(7) | 245(49) | 206/(41) | 581/(110) |
We also visited The Cao Vit Gibbon Conservation Area in Vietnam in June 2011. Although there were more than 10 groups living in this area, we were not permitted to observe every group and only recorded clear song bouts from three groups near a ranger station. Each group had one adult male (V1, V2, and V3 in Table 1). Vietnamese rangers in the Cao Vit Gibbon Conservation Area have monitored these three study groups for several years, so their home ranges are partially known.
Vocal Recording
We recorded song bouts in Bangliang from September 2007 to October 2012, excluding 2010 and in Trung Khanh during June 2011. Given that these gibbons normally produce song bouts in the early morning [32], we occupied the listening posts from 0600–1200 h. We recorded gibbon song bouts using a Sony TC-D5 Pro2 recorder with a Sony C-76 directional microphone and Sony analogue tapes (Sony C-90EFB and Sony C-60EFB).
Acoustic Analysis
All vocal recordings were digitized with a sample rate of 44.1 kHz and a sample size of 16 bits using the Sony TC-D5 Pro2 recorder and a 50 kHz Analog I/O Card (Engineer Design). The time-frequency sonograms and measurements of vocalizations were carried out using Signal/RTS 4.0 (Engineer Design). The FFT size of the sonograms was 1024 points with a frequency resolution of 43.1 Hz and a time resolution of 23.2 ms. Acoustic terminology used in this research (Table 2) follows that proposed by Konrad and Geissmann [33], Ruppell [34] and Feng et al. [35].
Table 2. Acoustic terms and definitions of gibbon song (following Konrad and Geissmann [33], Ruppell [34] and Feng et al. [35]).
| Term | Definition |
| Note | A single continuous sound of distinct frequency or frequency modulation that may be produced during either inhalation or exhalation |
| Roll | A characteristic of notes produced by the male wherein each roll includes a steep increase in frequency followed by a steep decrease |
| Phrase | A single vocal activity consisting of a larger or looser collection of notes or elements or both that may be produced together or separately |
| Male sequence | A complete sequence produced by the male, is made up of boom, aa note, pre-modulated note and modulated figure. The female dose not sing during this period |
| Song bout | All song notes of a gibbon group with periods of silence of <10 min |
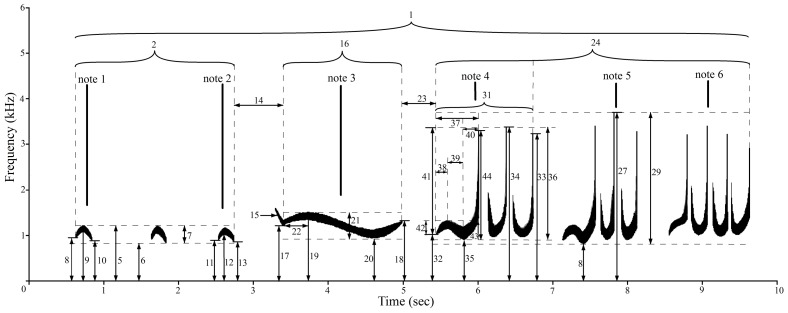
Recordings for which the sound quality was poor or the caller was uncertain were excluded. Male cao vit gibbons produced complex and differently structured phrases [35]. Male phrases responding to female great calls were excluded, because they are much more complex and differently structured from male phrases when females do not produce great calls, making it difficult to measure them using the same method [35]. We measured one of every six male phrases with five unmeasured phrases between two measured samples to increase independence between phrases. We measured the time and frequency of every inflection point of the fundamental frequencies of the male phrase including the first and last of the aa notes, pre-modulated notes and modulated figures (Fig. 1). We defined 44 variables based on the measurements to quantify the acoustic characteristics of the male phrase (Table 3). All measurements were done by Feng JJ to avoid interobserver differences.
Figure 1. Sonogram (only fundamental frequencies) of a fully male phrase showing most of the variables based on the measurements.
Table 3. Descriptions of acoustic variables and variables selected by 6 DFA analyses (“+” = Selected).
| No. | Part | Description | Individual differences | Acoustic stability | ||||
| 1st | 2nd | 3rd | 2007–2008 | 2007–2009 | 2007–2011 | |||
| 1 | Entire phrase | Total duration of entire phrase | + | + | + | |||
| 2 | aa notes | Total duration | + | + | ||||
| 3 | Number of notes | + | + | + | ||||
| 4 | Average duration of note1 and note2 | + | + | + | + | |||
| 5 | Highest frequency | + | + | + | + | + | + | |
| 6 | Lowest frequency | + | + | |||||
| 7 | Frequency range | + | + | |||||
| 8 | Note 1 | Start frequency | + | + | + | |||
| 9 | Middle frequency | + | + | + | ||||
| 10 | End frequency | + | + | + | ||||
| 11 | Note 2 | Start frequency | ||||||
| 12 | Middle frequency | |||||||
| 13 | End frequency | |||||||
| 14 | Time interval between note2 and note3 | + | + | + | ||||
| 15 | Note 3 | Occurrence of descending at the start | + | + | + | + | ||
| 16 | Duration | + | + | + | + | |||
| 17 | Start frequency | + | + | + | ||||
| 18 | End frequency | + | + | + | + | + | ||
| 19 | Highest frequency | + | ||||||
| 20 | Lowest frequency | + | + | + | + | + | ||
| 21 | Frequency range | + | + | + | + | |||
| 22 | Duration from start to the highest frequency point | + | + | + | + | + | + | |
| 23 | Time interval between note3 and note4 | + | ||||||
| 24 | Modulated figures | Total duration | ||||||
| 25 | Number of notes | + | + | + | + | |||
| 26 | Average duration of every note | + | + | + | + | |||
| 27 | Highest frequency | + | ||||||
| 28 | Lowest frequency | + | + | + | + | + | ||
| 29 | Frequency range | + | + | + | ||||
| 30 | Note 4 | Roll present or not | + | + | + | + | ||
| 31 | Duration | + | + | + | + | + | ||
| 32 | Start frequency | + | + | + | + | + | ||
| 33 | End frequency | + | ||||||
| 34 | Highest frequency | + | ||||||
| 35 | Lowest frequency | + | + | + | + | + | ||
| 36 | Frequency range | + | ||||||
| 37 | Initial part of Note 4 | Duration | + | + | + | + | ||
| 38 | Duration of initial part | + | ||||||
| 39 | Duration of middle part | + | + | + | ||||
| 40 | Duration of terminal part | + | + | + | + | + | + | |
| 41 | Frequency range of start to highest | |||||||
| 42 | Frequency range of initial part | + | + | + | + | + | ||
| 43 | Frequency range of middle part | + | + | + | + | + | + | |
| 44 | Frequency range of terminal part | + | ||||||
Variables repeatedly selected in all three individual difference analyses to test vocal individuality are bolded.
In total, we analyzed 581 phrases of 110 song bouts from 9 male individuals (Table 1); however, the sample size was not equal to 9 male individuals throughout 5 years, because of the absence of individuals in some years, and variable recording quality.
Statistical Analysis
We used stepwise discriminant function analysis (DFA) to identify individual differences and acoustic stability [1], [21], [22], [33], [36]. We chose Wilks' lambda as the criterion for variable selection. To test the significance of change in the selection criterion when we entered or removed a variable from a model, the probability of F was used and p-to-enter = 0.05 and p-to-remove = 0.10 were applied as significance levels [21], [22], [33]. To avoid empty cells in the data matrix, the start, middle, and end frequency of note 2 (No. 11, 12 and 13 in Table 3) were excluded. Thus 41 variables were chosen for every DFA. We did not replace the missing values with the mean.
In order to demonstrate individuality among the 9 males, a split-sample method was used to classify the remaining individuals [1], [22]. We randomly selected approximately 50% of phrases to calculate the discriminant functions, which we then used to reclassify the remaining samples. We repeated this process three times. In order to examine acoustic stability over time, a cumulative method was used to identify individuals in future years [22], [37]. We used phrases recorded in former years to calculate DFA functions and then used them to discriminate phrases in the following year. For example, DFA functions were calculated from phrases recorded between 2007–2008 and were used to discriminate phrases recorded in 2009. All statistical analyses were carried out using SPSS 16.0.
Results
Vocal Individuality
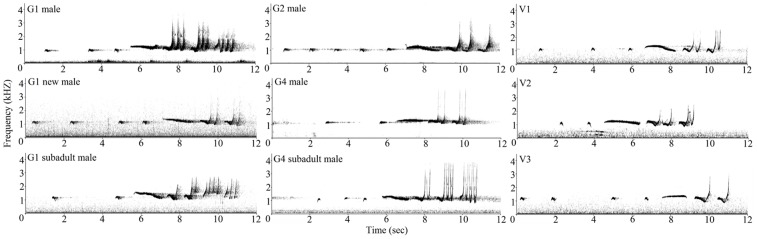
Of the 41 variables analyzed, 27, 30, and 26 variables were selected to classify phrases of individuals in 3 separate DFAs, and 19 variables were repeatedly selected by all 3 discriminant functions (Table 3). In each situation, DFA yielded a high correct rate of acoustic individual identification, even with the 19 repeatedly selected variables (Table 4). Typical sonograms of the 9 males are shown in Figure 2.
Table 4. The rate of correct individual recognition with different variables using DFA for male cao vit gibbons.
| Individuals | Correct rate (%) (41 variables) | Correct rate (%) (19 variables) | ||||
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | |
| G1 male | 93.1 | 96.2 | 90.0 | 97.8 | 97.3 | 97.8 |
| G1 new male | 94.3 | 92.6 | 100 | 85.2 | 96.7 | 84.8 |
| G1 subadult male | 95.6 | 96.0 | 91.5 | 93.6 | 90.0 | 97.7 |
| G2 male | 98.4 | 100 | 100 | 97.3 | 98.6 | 98.6 |
| G4 male | 91.8 | 82.9 | 84.8 | 89.1 | 77.3 | 83.7 |
| G4 subadult male | 81.2 | 75.0 | 85.7 | 90.0 | 75.0 | 100 |
| V1 | 100 | 100 | 100 | 100 | 100 | 100 |
| V2 | 100 | 100 | 100 | 100 | 100 | 100 |
| V3 | 95.8 | 95.0 | 100 | 96.7 | 96.3 | 96.3 |
| Mean | 94.7 | 94.2 | 94.0 | 94.4 | 92.9 | 94.5 |
Figure 2. Typical sonograms of the 9 adult male cao vit gibbons in this study.
Vocal Stability
Vocal stability was documented in all 4 males over 2–6 years (Table 5). For G1 male, the identification accuracy rates in 2009 and 2011 were 100%. And the DFA easily identified the male replacement in 2012 with the accuracy rate decreased to 23.9%. G2 male's vocalization remained very stable during the study and the correct rate was 100% in all years. Although the accuracy of discrimination was low for G4 male in 2011 and G1 subadult male in 2012, the majority of phrases were predicted to be from the correct individuals (Table 6).
Table 5. DFA classification results for male phrases of cao vit gibbons between years. G1 male was replaced by the G1 new male in 2012.
| Individuals | Discrimination | Discrimination | Discrimination | |||||||||
| 2007–2008 | 2009 | 2007–2009 | 2011 | 2007–2011 | 2012 | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| G1 male* | 50 | 100 | 4 | 100 | 54 | 100 | 11 | 100 | 51 | 100 | 46 | 23.9 |
| G2 male | 32 | 100 | 38 | 100 | 70 | 100 | 56 | 100 | 126 | 100 | 8 | 100 |
| G4 male | 6 | 100 | 54 | 51.9 | 59 | 100 | 30 | 100 | ||||
| G1 subadult male | 15 | 100 | 80 | 77.5 | ||||||||
Table 6. DFA classification results for male phrases of G4 male in 2011 and G1 subadult male in 2012.
| Individuals | Predicted group membership | |||||||
| G1 male | G1 subadult male | G2 male | G4 male | |||||
| n | % | n | % | n | % | n | % | |
| G4 male | 13 | 24.1 | 13 | 24.1 | 28 | 51.9 | ||
| G1 subadult male | 62 | 77.5 | 10 | 12.5 | 8 | 10.0 | ||
Discussion
In this study, we provide the first demonstration of vocal individuality and stability in male songs of cao vit gibbons. Discriminant function analysis with 19 variables was able to classify all 9 males studied, and in most cases, male songs recorded from former years were able to identify individuals recorded in the following year. Due to the international border splitting the nature reserve and the very small gibbon population in China, we could record only 9 individuals to test vocal individuality and 4 individuals to test vocal stability over the 6 years' study. While we acknowledge the small sample size in this study, our results are valuable given the extraordinary difficulty of studying and conserving this critically endangered species. Given that the entire species is comprised of fewer than 20 predominantly inaccessible groups [25], identification of vocal individuality, although imperfect, will likely provide the only repeatable monitoring data for cao vit gibbons.
In two situations when we used phrases recorded from previous years to identify phrases in the following year, the identification accuracy was low. One possible explanation is that the number of phrases used to calculate the discriminant functions was much smaller than the number of phrases waiting for classification (G4 male in 2011 and G1 subadult male in 2012). Studies have shown that vocal features can vary with social context in birds [38], dolphins [39], and primates [40], [41]. Gibbons may also change their calls in response to differences in social context, such as visual contact with neighboring groups or losing contact with group members. These changes might obscure vocal individuality [42]. Given the reality of this variation, the greater the number of phrases used to calculate the functions, the more powerful the functions would likely be. In the other six situations in which the number of phrases used to calculate functions was larger or roughly equivalent to the number of phrases waiting for classification, the classification accuracy was 100%. After we increased the number of phrases used to calculate the discriminant functions, the classification accuracy of G4 male in 2012 also increased to 100%. Another reason might be that gibbons may change their vocalization during development, which has been documented in female gibbons [43]. Based on our own observations, subadult male cao vit gibbons produce relatively simple structured phrases when they begin to sing solo bouts that develop into complicated adult male phrases after intensive practice for 1–2 years.
As we demonstrate here, using discriminant function analysis to identify individual male gibbons by their unique vocalizations has multiple uses. Currently, the triangulation method developed by Brockelman and Srikosamatara [8] is widely used to survey gibbon populations [44]–[48]; however, when different groups in close proximity sing at different times, it can be difficult to obtain an accurate population estimate [47]. By combining triangulation with individual recognition using vocal individuality in male songs, we may be able to obtain more accurate estimates of population size, which are crucial for the conservation of small populations. We can also use vocal recording to monitor population dynamics in the cao vit gibbon population. These gibbons live in relatively stable territories and remain in the same territory after male replacement (unpublished data). If an adult male is replaced by a new male, DFA could be used to reliably tell them apart. Indeed, we observed one male replacement during our research, and it was confirmed using vocal identification. However, several practical issues should be considered before implementing this technique more broadly.
Gibbon songs should be recorded in the optimal season: Cao vit gibbons sing more regularly during the rainy season, especially in between July and September (unpublished data). We suggest recording gibbon songs during this period.
Record multiple bouts to identify individuals when possible: Paired males produce solo or duet bouts with females lasting on average 18.3 min [32], and adult males producing diverse phrases during each bout. While we have demonstrated that one bout is usually enough to identify an individual, two or more bouts are highly recommended if time and labor are available.
Gibbons should be recorded once per year: Male tenure is quite long in gibbons [49], and in this population, male tenure could last more than 6 years. As a result, recording male songs once every year should be enough to document male replacements, subadult male dispersals, and group loss. Although we were not able to test whether the vocalizations of subadult males remain stable after dispersal, Fan et al. reported that a western black crested gibbon (the closest relative of cao vit gibbons [50]) subadult male did not change its song after it replaced a neighboring group's male [22].
Cooperation between China and Vietnam is needed: Considering the sole remaining population of this species crosses the international border between China and Vietnam, it is difficult to implement any conservation or research activities without cooperation between these countries. Both subadult males and females disperse freely across the border [29] but people cannot. Vocal recording methods could be the only way to detect dispersal patterns uniformly in this transboundary population.
In conclusion, we have detected vocal individuality and stability in the critically endangered cao vit gibbons. Facilitated by their regular and loud morning songs, stable territories, and long-term male tenure, using vocal individuality to monitor gibbon population dynamics is feasible. Given the difficult terrain and international border that prevents tracking the dispersal of individuals in this area, the identification of vocal individuality could be the only method to detect dispersal patterns in this population. Furthermore, this time- and labor-saving method could be standardized easily in both China and Vietnam.
Acknowledgments
We thank Mr. Tan Wujin, Huang Tao, Lin Yucong, and Yang Xiao from the Jingxi Forestry Bureau for their needed support. We are grateful to Yu Chonghao and Huang Tianzhu for their help during fieldwork. We are grateful to Dr. Joseph Orkin and three anonymous reviewers for their valuable comments and English edit.
Funding Statement
This research was funded by the National Natural Science Foundation of China (# 30900169, www.nsfc.gov.cn), Conservation Leadership Programme (060208, F0237110, http://www.conservationleadershipprogramme.org/), Fauna and Flora International (http://www.fauna-flora.org), and Association of Zoos and Aquariums (www.aza.org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Oyakawa C, Koda H, Sugiura H (2007) Acoustic features contributing to the individuality of wild agile gibbon (Hylobates agilis agilis) songs. American Journal of Primatology 69: 777–790. [DOI] [PubMed] [Google Scholar]
- 2. Tibbetts EA, Dale J (2007) Individual recognition: it is good to be different. Trends in Ecology and Evolution 22: 529–537. [DOI] [PubMed] [Google Scholar]
- 3. Haimoff EH, Gittins SP (1985) Individuality in the songs of wild agile gibbons (Hylobates agilis) of Peninsular Malaysia. American Journal of Primatology 8: 239–247. [DOI] [PubMed] [Google Scholar]
- 4. Butynski TM, Chapman CA, Chapman LJ, Weary D (1992) Use of male blue monkey “pyow” calls for long-term individual identification. American Journal of Primatology 28: 183–189. [DOI] [PubMed] [Google Scholar]
- 5. Puglisi L, Adamo C (2004) Discrimination of individual voices in male great bitterns (Botaurus stellaris) in Italy. The Auk 121: 541–547. [Google Scholar]
- 6. Tripp TM, Otter KA (2006) Vocal individuality as a potential long-term monitoring tool for Western Screech-owls, Megascops kennicottii . Canadian Journal of Zoology 84: 744–753. [Google Scholar]
- 7. Galeotti P, Pavan G (1991) Individual recognition of male tawny owls (Strix aluco) using spectrograms of their territorial calls. Ethology Ecology & Evolution 3: 113–126. [Google Scholar]
- 8. Brockelman WY, Srikosamatara S (1993) Estimating density of gibbon groups by use of the loud songs. American Journal of Primatology 29: 93–108. [DOI] [PubMed] [Google Scholar]
- 9. Terry AMR, Peake TM, McGregor PK (2005) The role of vocal individuality in conservation. Frontiers in Zoology 2: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lok CBP, Tan XF, Tan WJ (2008) Rediscovery of the critically endangered eastern black crested gibbon Nomascus nasutus (Hylobatidae) in China, with preliminary notes on population size, ecology and conservation status. Asian Primates Journal 1: 17–25. [Google Scholar]
- 11. Xia CW, Lin XL, Liu W, Lloyd H, Zhang YY (2012) Acoustic identification of individuals within large avian populations: a case study of the brownish-flanked bush warbler, south-central China. PLoS ONE 7: e42528 doi: 10.1371/journal.pone.0042528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leighton DR (1987) Gibbons: territoriality and monogamy. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors.Primate societies. Chicago:The University of Chicago Press. pp. 135–145
- 13. Geissmann T, Orgeldinger M (2000) The relationship between duet songs and pair bonds in siamang, Hylobates syndactylus . Animal Behaviour 60: 805–809. [DOI] [PubMed] [Google Scholar]
- 14. Geissmann T (2002) Duet-splitting and the evolution of gibbon songs. Biological Reviews 77: 57–76. [DOI] [PubMed] [Google Scholar]
- 15. Cowlishaw G (1992) Song function in gibbons. Behaviour 121: 131–153. [Google Scholar]
- 16. Fan PF, Xiao W, Huo S, Jiang XL (2009) Singing behavior and singing functions of black crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, Central Yunnan, China. American Journal of Primatology 71: 539–547. [DOI] [PubMed] [Google Scholar]
- 17. Mitani JC, Gros-Louis J, Macedonia JM (1996) Selection for acoustic individuality within the vocal repertoire of wild chimpanzees. International Journal of Primatology 17: 569–583. [Google Scholar]
- 18. Haimoff EH, Tilson RL (1985) Individuality in the female songs of wild Kloss' gibbons (Hylobates klossii) on Siberut Island, Indonesia. Folia Primatologica 44: 129–137. [Google Scholar]
- 19. Dallmann R, Geissmann T (2001) Different levels of variability in the female song of wild silvery gibbons (Hylobates moloch). Behaviour 138: 629–648. [Google Scholar]
- 20. Dallmann R, Geissmann T (2001) Individuality in the female songs of wild silvery gibbons (Hylobates moloch) on Java, Indonesia. Contributions to Zoology 70: 41–50. [Google Scholar]
- 21. Sun GZ, Huang B, Guan ZH, Geissmann T, Jiang XL (2011) Individuality in male songs of wild black crested gibbons (Nomascus concolor). American Journal of Primatology 73: 431–438. [DOI] [PubMed] [Google Scholar]
- 22. Fan PF, Xiao W, Feng JJ, Scott MB (2011) Population differences and acoustic stability in male songs of wild western black crested gibbons (Nomascus concolor) in Mt. Wuliang, Yunnan. Folia Primatologica 82: 83–93. [DOI] [PubMed] [Google Scholar]
- 23.IUCN (2012) The IUCN Red List of Threatened Species. http://www.iucnredlist.org.
- 24.Long YC, Nadler T (2009) Eastern black crested gibbon Nomascus nasutus (Kunkel d'Herculais, 1884), China, Vietnam. In Primates in Peril: The World's 25 Most Endangered Primates 2008–2010. In: Mittermeier RA, Wallis J, Rylands AB, Ganzhorn JU, Oates JF, Williamson EA, Palacios E, Heymann EW, Kierulff MCM, Long YC, Supriatna J, Roos C, Walker S, Cortés-Ortiz L, Schwitzer C, editors.Arlington, IUCN/SSC Primate Specialist Group, International Primatological Society and Conservation International. pp. 60–61.
- 25.Le TD, Fan PF, Yan L, Le HO, Josh K (2008) The global cao vit gibbon (Nomascus nasutus) population. Unpublished report to Fauna & Flora International, Vietnam Programme and China Programme.
- 26. Fan PF, Fei HL, Ma CY (2012) Behavioral responses of cao vit gibbon (Nomascus Nasutus) to variations in food abundance and temperature in Bangliang, Jingxi, China. American Journal of Primatology 74: 632–641. [DOI] [PubMed] [Google Scholar]
- 27. Mootnick AR, Fan PF (2011) A comparative study of crested gibbons (Nomascus). American Journal of Primatology 71: 1–20. [DOI] [PubMed] [Google Scholar]
- 28. Fan PF, Ren GP, Wang W, Scott MB, Ma CY, et al. (2013) Habitat evaluation and population viability analysis of the last population of cao vit gibbon (Nomascus nasutus): Implications for conservation. Biological Conservation 161: 39–47. [Google Scholar]
- 29. Fan PF, Jiang XL, Liu CM, Luo WS (2010) Sonogram structure and timing of duets of western black crested gibbon in Wuliang Mountain. Zoological Research 31: 293–302. [DOI] [PubMed] [Google Scholar]
- 30. Fan PF, Fei HL, Scott MB, Zhang W, Ma CY (2011) Habitat and food choice of the critically endangered cao vit gibbon (Nomascus nasutus) in China: implications for conservation. Biological Conservation 144: 2247–2254. [Google Scholar]
- 31. Fei HL, Scott MB, Zhang W, Ma CY, Xiang ZF, et al. (2012) Sleeping tree selection of cao vit gibbon (Nomascus nasutus) living in degraded karst forest in Bangliang, Jingxi, China. American Journal of Primatology 00: 1–8. [DOI] [PubMed] [Google Scholar]
- 32. Fei HL, Fan PF, Xiang ZF, Ma CY, Zhang W, et al. (2010) Effect of meteorological factors on singing behavior of eastern black crested gibbons (Nomascus nasutus). Acta Theriologica Sinica 30: 377–383. [Google Scholar]
- 33. Konrad R, Geissmann T (2006) Vocal diversity and taxonomy of Nomascus in Cambodia. International Journal of Primatology 27: 713–745. [Google Scholar]
- 34. Ruppell J (2010) Vocal diversity and taxonomy of Nomascus in central Vietnam and southern Laos. International Journal of Primatology 31: 73–94. [Google Scholar]
- 35. Feng JJ, Ma CY, Fei HL, Cui LW, Fan PF (2013) Call sonograms of eastern black crested gibbon (Nomascus nasutus). Acta Theriologica Sinica 33: 203–214. [Google Scholar]
- 36. Jones BS, Harris DHR, Catchpole CK (1993) The stability of the vocal signature of phee calls of the common marmoset, Callithrix jacchus . American Journal of Primatology 31: 67–75. [DOI] [PubMed] [Google Scholar]
- 37. Klenova AV, Volodin IA, Volodina EV (2009) Examination of pair-duet stability to promote long-term monitoring of the endangered red-crowned crane (Grus japonensis). Journal of Ethology 27: 401–406. [Google Scholar]
- 38. Park SR, Park D (2000) Song type for intrasexual interaction in the bush warbler. The Auk 117: 228–232. [Google Scholar]
- 39. Janik VM, Dehnhardt G, Todt D (1994) Signature whistle variation in a bottlenose dolphin, Tursiops truncatus . Behavioral Ecology and Sociobiology 35: 243–248. [Google Scholar]
- 40. Elowson AM, Snowdon CT (1994) Pygmy marmosets, Cebuella pygmaea, modify vocal structure in response to changed social environment. Animal Behaviour 47: 1267–1277. [Google Scholar]
- 41. Mitani JC, Brandt KL (1994) Social factors influence the acoustic variability in the long-distance calls of male chimpanzees. Ethology 96: 233–252. [Google Scholar]
- 42. Fox EJS (2008) A new perspective on acoustic individual recognition in animals with limited call sharing or changing repertoires. Animal Behaviour 75: 1187–1194. [Google Scholar]
- 43. Koda H, Lemasson A, Oyakawa C, Rizaldi, Pamungkas J, et al. (2013) Possible role of mother-daughter vocal interactions on the development of species-specific song in gibbons. PLoS ONE 8: e71432 doi: 10.1371/journal.pone.0071432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buckley C, Nekaris KAI, Husson SJ (2006) Survey of Hylobates agilis albibarbis in a logged peat-swamp forest: Sabangau catchment, Central Kalimantan. Primates 47: 327–335. [DOI] [PubMed] [Google Scholar]
- 45. Jiang XL, Luo ZH, Zhao SY, Li RZ, Liu CM (2006) Status and distribution pattern of black crested gibbon (Nomascus concolor jingdongensis) in Wuliang Mountains, Yunnan, China: implication for conservation. Primates 47: 264–271. [DOI] [PubMed] [Google Scholar]
- 46. Phoonjampa R, Brockelman WY (2008) Survey of pileated gibbon Hylobates pileatus in Thailand: populations threatened by hunting and habitat degradation. Oryx 42: 600–606. [Google Scholar]
- 47.Brockelman WY, Naing H, Saw C, Moe A, Linn Z, et al. (2009) Census of eastern hoolock gibbons (Hoolock leuconedys) in Mahamyaing wildlife sanctuary, Sagaing Division, Myanmar. In: Lappan S, Whittaker DJ, editors.The Gibbons: New Perspectives on Small Ape Socioecology and Population Biology. Springer, New York. pp. 435–452.
- 48. Fan PF, Xiao W, Huo S, Ai HS, Wang TC, et al. (2011) Distribution and conservation status of Hoolock leuconedys in China. Oryx 45: 129–134. [Google Scholar]
- 49. Brockelman WY, Reichard U, Treesucon U, Raemaekers JJ (1998) Dispersal, pair formation and social structure in gibbons (Hylobates lar). Behavoural Ecology and Sociobiology 42: 329–339. [Google Scholar]
- 50. Thinh VN, Rawson B, Hallam C, Kenyon M, Nadler T, et al. (2010) Phylogeny and distribution of crested gibbons (genus Nomascus) based on mitochondrial cytochrome b gene sequence data. American Journal of Primatology 72: 1047–1054. [DOI] [PubMed] [Google Scholar]