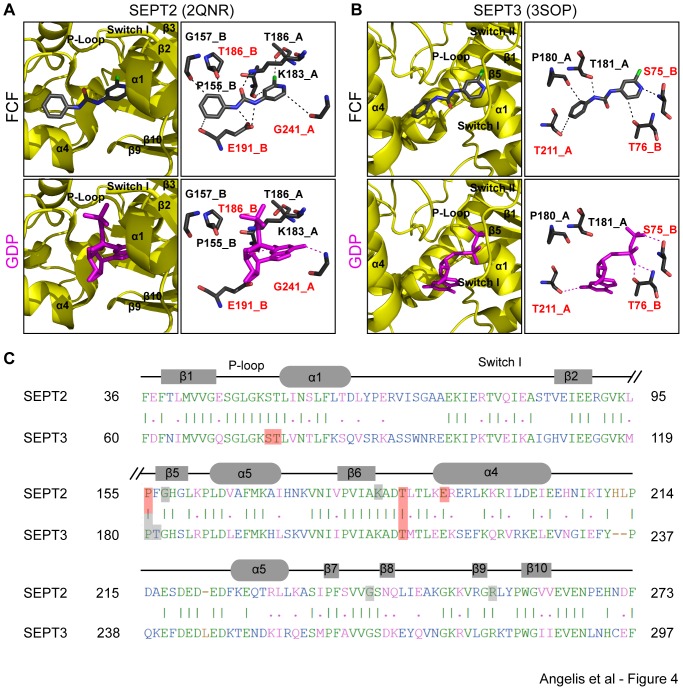
Figure 4. FCF is predicted to interact preferentially with the nucleotide-binding pockets of SEPT2 and SEPT3.
(A and B) Ribbon and stick representations show the most energetically favorable conformations of FCF bound to SEPT2 (A) and SEPT3 (B) compared to the crystal structures of the GDP-bound SEPT2 (PDB: 2QNR) and SEPT3 (PDB: 3SOP). Ribbon representations show the position of FCF and GDP with respect to the alpha helices, beta strands, P-loop, and the switch I and switch II regions of the nucleotide-binding pocket of septins. Stick representations depict the amino acids and atomic bonds (dotted lines) underlying the septin interactions with FCF and GDP. Red text denotes amino acids and their corresponding protomers that interact with both FCF and GDP. (C) Alignment of the amino acid sequences of SEPT2 and SEPT3. Common residues that interact with both FCF and GDP are shaded in red and all other amino acids that interact with FCF are shaded in gray. Identical and similar amino acids are shown in green and pink fonts, respectively. Sequence mismatches and insertions/deletions are denoted in blue and brown, respectively.