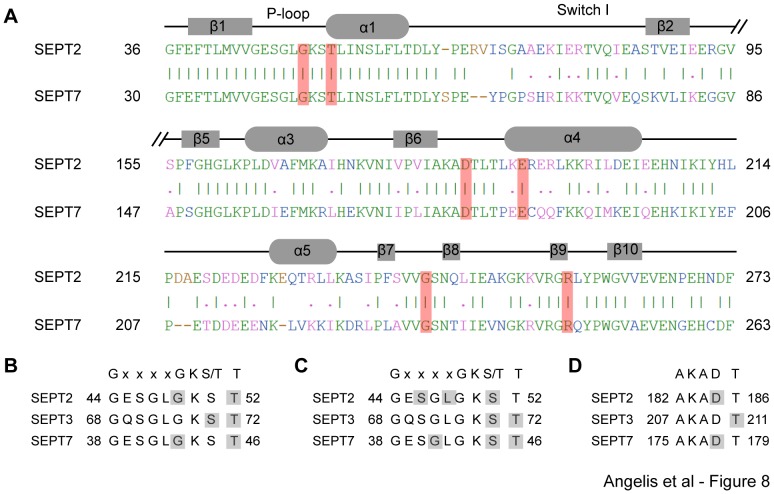
Figure 8. In silico FCF interacts with highly conserved septin residues and signature motifs.
(A) Sequence alignment highlights the conserved amino acids (shaded in red) that mediate the interaction of FCF with the deep end of the nucleotide binding pockets of SEPT2 (PDB: 3FTQ) and SEPT7 (PDB: 3T5D). Identical and similar amino acids are shown in green and pink fonts, respectively. Sequence mismatches and insertions/deletions are denoted in blue and brown, respectively. (B and C) Sequence alignments of the Walker A motif GxxxxGKS/T and the immediately following threonine, an invariant septin residue, highlight the amino acids (shaded in gray) that interact with FCF at the deep end (B) and outer side (C) of the nucleotide-binding pockets of SEPT2 (PDB: 3FTQ), SEPT3 (PDB: 3SOP) and SEPT7 (PDB: 3T5D). (D) Sequence alignments of the GTP-binding specificity motif AKAD and the following threonine residue highlight the amino acids (shaded in gray) that interact with FCF at the deep end of the nucleotide-binding pockets of SEPT2 (PDB: 3FTQ), SEPT3 (PDB: 3SOP) and SEPT7 (PDB: 3T5D).