Figure 6. Effects of URB597 and WIN 55,212-2 treatment over seven days on below-level cutaneous hypersensitivity in rats with neuropathic SCI pain.
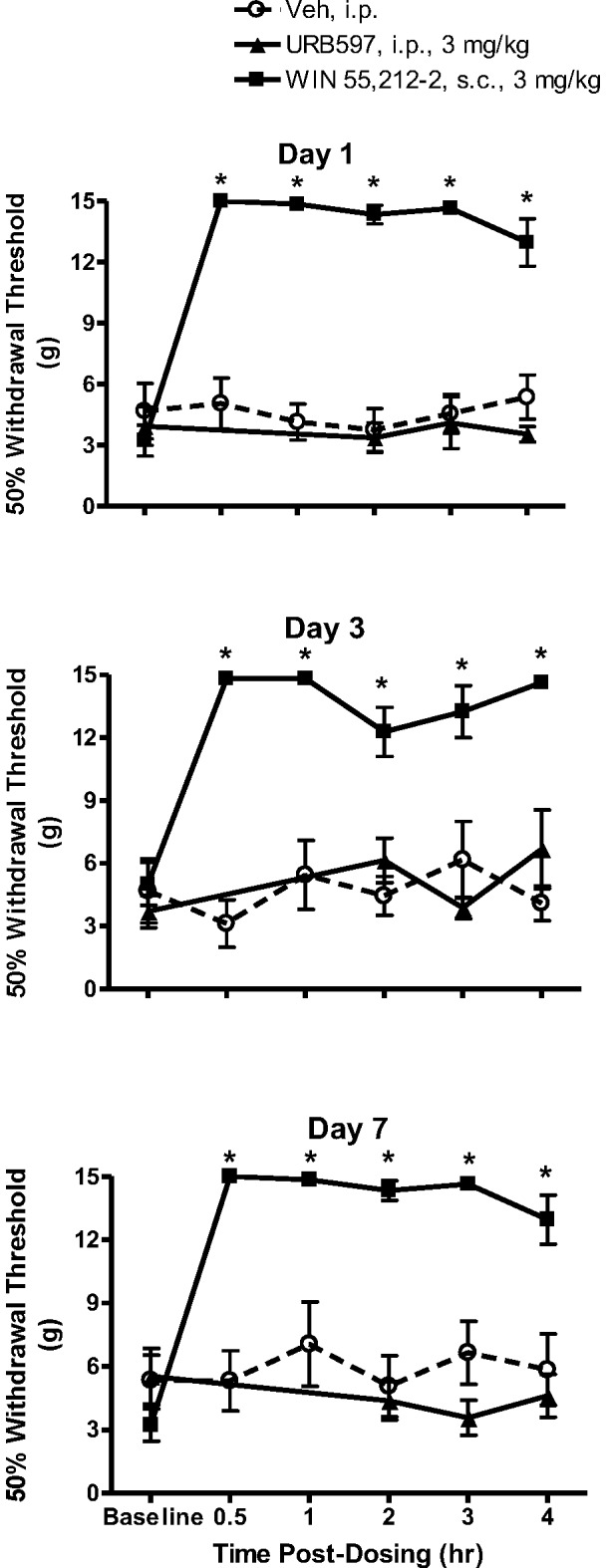
Baseline hind paw withdrawal thresholds were measured prior to treatment with either URB597 (3 mg/kg, i.p.), WIN 55,212-2 (3 mg/kg, s.c.) or vehicle (Veh, 1.5 ml/kg, i.p.). Rats were treated twice daily and tested following the first daily injection. On the first day of testing, a robust antinociception was observed beginning 30 min post-injection of WIN 55,212-2, which was observed also observed on days 3 and 7. By contrast, no antinociceptive effects were observed following treatment with either URB597 or vehicle. Data presented as mean ± S.E.M. n = 8–10/group. * p<0.05 vs. vehicle (Two-way repeated measures ANOVA, Student-Newman-Keuls test).
